62
62dotted lines are the time intervals.... (I mean generally one signal)
 106
106yes sir,,, the comprehension was from physics...
and I too feel taht the answer should be negative....
REASON:
In phy... work done by gas in expansion is positive
work done in compression by gas --- negative
work done on gas in expansion ----- negative
work done on gas in compression ---- positive
reason for these statements
In phy W = F.dx
in expansion F.dx > 0 ,F = F of gas .. so work done by gas > 0
in expansion F.dx < 0 , F=F(ext) .. so, work done on gas < 0
In compression F.dx < 0, F = F of gas .. so work done by gas < 0
In compression, F.dx > 0, F = F(ext). .. So work done on gas > 0
hope im correct in these statements..
 1357
1357Sign Conventions are too confusing
If you go by physics, work done by system is taken as positive.
So according to that the answer in q 34 should be negative
 106
106thanks sir
btw ....
why is no one trying to even think about what I have written about the signs of the compre. question..... I know what we assume in chem AS WELL AS what we assume in phy ..regarding signs...
I am just asking ppl to read the question a bit more carefully and answer accordingly giving full justifiable answers...
 1357
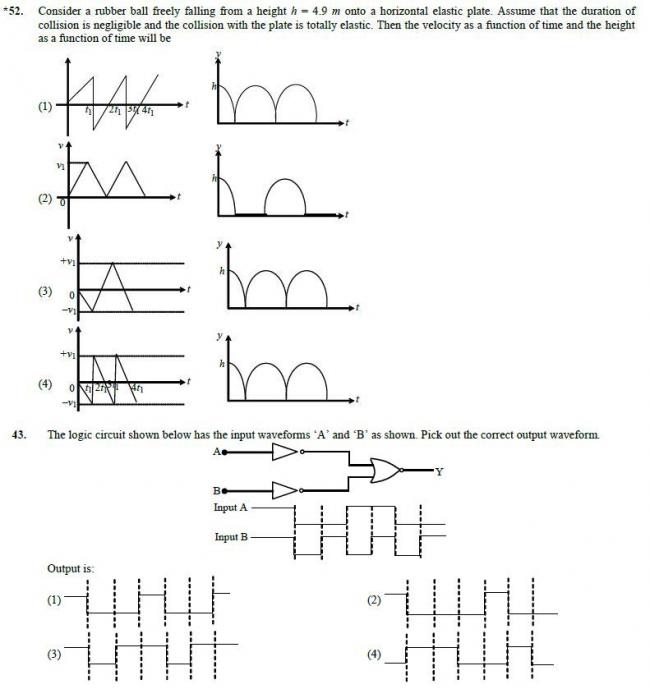
1357For (52)
In option (4) If I differentiate second graph(y vs t) it gives me the first graph(v vs t)
Regarding direction:
It is clear from second graph that y is decreasing, so +ve axis is taken upward
 1
1read the ncert text book for physics...explanation is given how to solve the problems related to signals.
 1
1-ve in expansion for chemistry thermo not physics thermo isliye ...
 1
1@ Asish i thnk lyman corresponds to ultraviolet .
how u said infrared culd be achieved with 2→1 transisition ??
 1
152)@ashish observe carefully in (1) the graph comes down straight at t1,3t1. so it is wrong as it cannot have two velocities at the same time.
in (4) the graph is slightly tilted at those points making it correct.
it is nothing abt direction.that way both shud hav been correct
 106
106k sir... thanks
btw can u tell abt the signs of that phy comprehension question ? 34-36 ?
 1
143) is easy just write the truth table which is as
A B Output
0 0 0
0 1 0
1 0 0
1 1 1
now according to this graph is (2)
solid lines are as to the down states 0 output only inflexion in (2) shows 1 output.
that's wat i thnk but ya it's not clear though.
abt the 52) one not sure . so sry for that
 62
62dotted lines are the time intervals.... (I mean generally one signal)
 106
10652. still unsure abt direction
43. solved yeah could be
55.. solved@mkm .. i know that logic..(even acc to that we still dont have guarantee that delta E will be low enough to give infrared waves)
but dont we get infrared for lyman series?? so shouldnt(2) be the answer also (assuming that (1) is correct)
84. im asking abt delta V...
as V = 600m/s ± 0.005%
Shouldnt delta V = 2*0.005%*600 m/s (as total variation is this much?)
34 work ON the gas (as it is expansion) is -ve naa?
35 work ON the gas (as it is compression) is +ve naa?
 1
152.> I THINK IT SHUD BE (4)
AS THE BALL IS FALLIN IT SHUD HAV -VE INITIAL VELOCITY
@MKM..IF I FOLLOOW UR METHOD IN Q43 THEN ..I AM GETTIN (B) AS ANS
 39
39Manmay as long as his tables say they ain't wrong, they ain't wrong :P
 1
1@asish don't u thnk there was a mistake in that chemistry Ionic radius wala question in AIEEE 2009[11]
 1
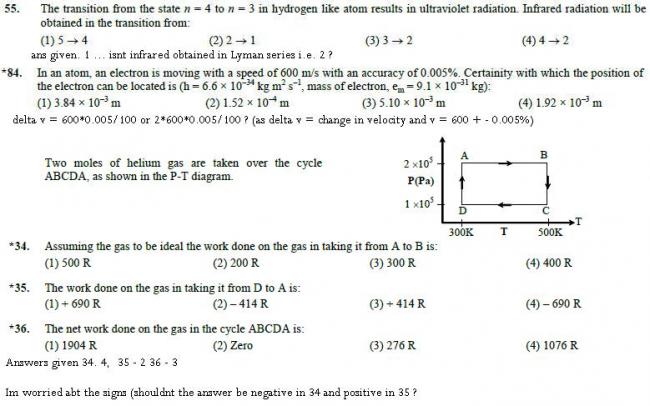
134)
Process A - B → pressure is constant
so W = PΔV → nRΔT → 2 x R ( 500 - 300 ) = 400 R
35)
D - A → temperature is constant
so W = 2.303 nRT log (VAVD) → 2.303 nRT log (PDPA) → 2.303 x 2 x R x 300 log 1052 x 105
→ - 1381.8R log 2 = - 415.92R ≈ -414 R according to option nearest
36)
W net = W A-B + WB-C + WC-D + W D-A
W CD = nRΔT= - 400 R
W BC = 2.303 nRT log (PBPC) = 693.2R
hence Wnet = 400R + 693.2 R - 400R - 414 R = 279.2R ≈ 276 R according to options
 1
184)
Δx . ΔV = h4πm
→ Δx . 600 x 0.005100 = 6.62 x 10-344 x 3.14 x 9.1 x 10-31
→ Δx = 1.92 x 10-3 m
 1
155 ) for infrared wavelength shuld be low hence ΔE shuld be more which is for 5→4 transition i guess