 106
106hmm. okay..
ill show you my pKa table..
This ones validated by all Sr. Members of chemicalforums.com
 29
29@Arsh..see dude i too got confused abt it...see my post #13..
and for ur own question...both the compounds have 3methy grps attached to Carbon?
then choloro isomer will have more dipole moment because of bond length is large in this case..and it's the governing factor..well this kinda things are exception..but thanks to ncert they have given a table on dipole moment which proves the answer in alkyl halide chapter..
or pKa values bhi OH wale ki zyada hain matlab OH wala zyada acidic hian
no..the compound having larger pKa is less acidic
 24
24@arsh...
OH is more activating that OR...
http://en.wikipedia.org/wiki/Activating_group
but main question still not solve...Q1
 1
1i think o-R is more acticvating than OH. pankaj ne notes mein likhaya tha. par haan wo kahan sahi likhata hain. mereko explanation nahi pta. par note copy mein mereko O-R > OH likha hua hain.
or pKa values bhi OH wale ki zyada hain matlab OH wala zyada acidic hian so O-R wale ka resonating effect zyada hoga matlab O-R zyada activating hain. (provided jo values asish ne likhi hian woh sahi ho)
 1
1dear friend
you were the one who added the value of pka in the 1st spot. thouhg your explanation for it was very very lousy. electron electron replusion and high electron gain enthalpy will be accounted for when it goes into an anion.
well may be the answer can be solved while we answer this question. can anyone please explain the dipole moment in these two compounds? &
& 
the answer is that the chlorine isomer has more dipole moment. how will your explain this. i have my own theory but it is a theory only. can you all please try and explain. this will lead to the answer of the 1st question.
 24
24i dont know dude...i only have the ans
 1
1@saket can u please assert why its 9???
 21
21can somebody give the answers to first one.....is it 9?
 1
1ok whatever.....whether Pka values or what so ever.....that must have a logic.....as i explained in #11 either agree or disagree...in either case give an explanation of which is corect!!!!!
 106
106arey kya mujhe pataa nahiin yeh sab...
im NOT Swami Vivekananda..
I hope such confusing questions really dont come in JEE.. as said by Pritish above..
I actually wrote another answer before posting, then deleted as i got really confused and searched pKa values
but there is one good aspect..
If you have a list as this.. you can use the values to organise ur thinking (invent weird reasons as explanations) and hope that this thing strikes u in exam as well
 39
39That's a splendid idea Asish...but using facts that are established during a competitive exam is a non-bailable offence which can destroy your candidature. Hope you read the exam rules...if not I'll quote them for your convenience -:

Sadly for JEE we need cold, cruel logic and nothing else. If our heads stop working there, nobody can save us.
Of course, if your mind is as strong and versatile as Swami Vivekananda's that you can remember the pKas of every damn organic compound that was synthesised or discovered, hats off to you. Phir kya kehna.
 106
106i know, but when heads start giving wrong answers..
waht r u supposed to do????
LOOK AT FACTS THAT HAVE BEEN ESTABLISHED
 39
39Waise why are you doing this question by pKas? I was only trying to verify the acidity order...
Aren't we supposed to use our heads?
 39
39Asish I searched the pKas on the net.
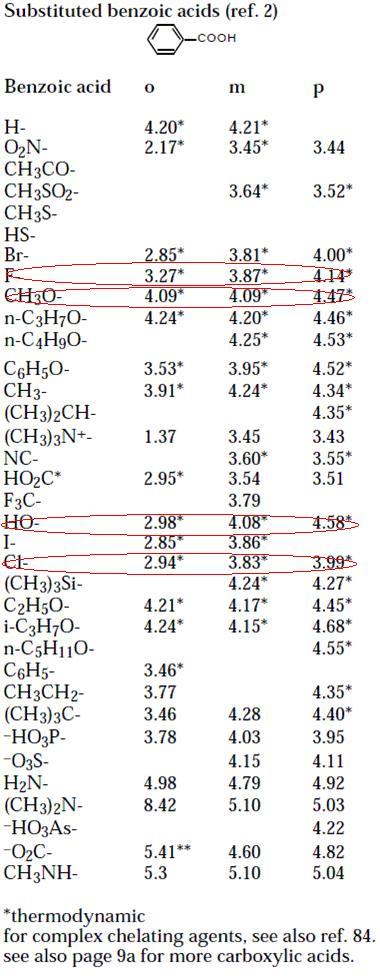
p-hydroxybenzoic acid : 4.48
p-methoxybenzoic acid : EXPECTED pKa is 4.48, ACTUAL pKa is 4.82
 106
106@gagar bhaiya: can you show how 10 is the answer?
 106
106govind: I showed you pKa values naa?
btw if you want, you can also search in Morrison Boyd (It should be there)
 29
29I have a dbt in this question..
i think UTTARA' answer in post #2 shud be correct..
edited: sry guys for creating the confusion..i agree with Asish's answer ...
Final Conclusion : OH is more activating than OCH3
 39
39p-flourobenzoic acid pKa ≈ 4
p-chlorobenzoic acid pKa ≈ 3
Judge for yourself :P
Actually, flourine loses out because it has less negative electron gain enthalpy than chlorine, because of its small size and high electron-electron repulsion.
 1
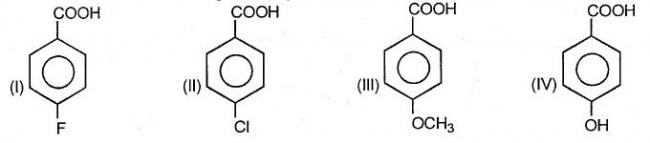
1for Q2) not only fluorine has max -i effect but also Cl has a slight tendency of +m which fluorine doesn't so Cl will will make the ring less electron deficient than F.....threrefore...the order should be
1>2>4>3
 1
1@gagar,asish can anybody explain how u are getting 10 or isomers???
 106
106i was doing .. the double bonds can be EE, ZZ, EZ - 3
similarly, RS can be RR, RS, SS - 3
So total 9
 24
24Q1 koi karo ...
@pranav...take care of resonance effect too
 1
1greater than cl na??plzz tell me if i am wrong !!
 1
1is shud be 1 > 2>4>3....as F has max -I effect na??
 24
24thx for 2..had dbt b/w I and II
btw for Q1 ans is 10


 &
& 