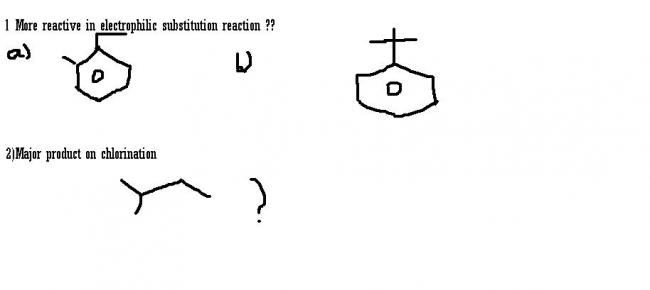
for 1....(b)......in first both groups are antagonistic to each other ..
7 Answers
Asish Mahapatra
·2010-01-26 18:31:32
2. use the principle of reactivity.
abstraction of 2° H is 3.8 and for 1° H = 1 and for 3° H = 5
then calculate relative percentage for the various products possible
eg. for 1chloro2methylbutane it would be 6*1/(total sum)
for 2chloro2methyl ... it wud be 1*5/(total)
for 2chloro3methyl ... it wud be 2*3.8/(total)
for 1chloro 3methyl it wud be 1*3/(total)
So u can see max is for 2chloro3methylbutane
For 1. there are two ERG in (a) while only 1 ERG in (b) further as tert. butyl is quite large, so delocalisation due to hyperconj. is minimised...
But in (a) there are 5 Hyperconj Hydrogens
So (a) is more reactive