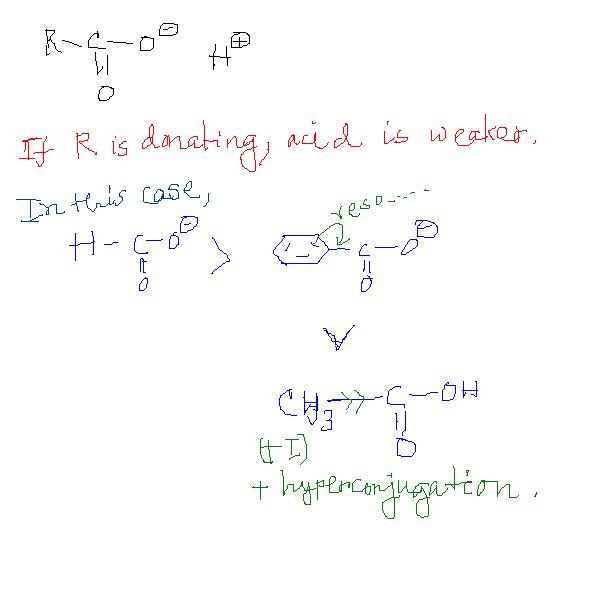Hey..... any one plz......
Why C6H5COOH is less acidic than HCOOH, but more acidic than CH3COOH?
-
UP 0 DOWN 0 1 11

11 Answers
Because HCOOH is more acidic than CH3COOH
But don' know how PhCOOH comes in between them
Why does PhCOOH come in between them??......i want to know exactly that
* if R is donating then acid weaker...
because... the conjugate base will be weaker...
Sahi hi lag raha hai..
But Resonance must increase more e- than +I and Hyper..
As in CH3-CH2+ and Ph-CH2+
But the order is correct ...
here I think Ph stops delocalisation of - charge on -C=O
|
O-
Due to cross conjugation..
yeh cross-conjugation ka kya chakkar hai ??
i jus thot, here there are one effect n in methyl grp there are two effects ...
And for cross conj :D
a very handy tool of teacher to explain any exception :D
cross conj, steric effect one of d most powerful tool 4 them [6]
sp2 hybridised carbon is more basic than that of the sp3 hybridised carbon so the cpd C6H5COOH is more basic than that of CH3COOH
yeah abhisekh .. u are r8..
kuchh na ho toh...... steric hindrance :P :P