the increaisng tendency towards esterification is .
B<C<A
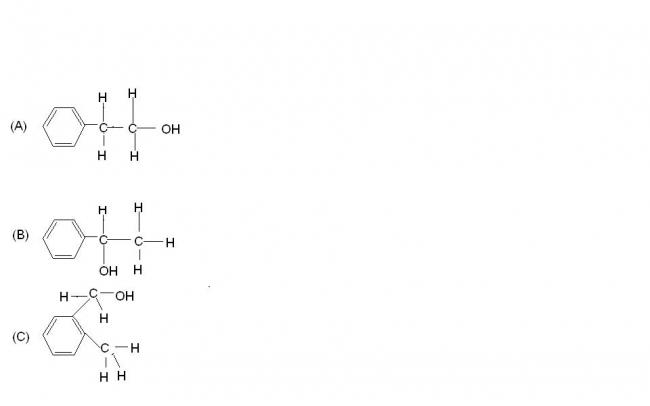 arrange the following alcohols in the increasing order of tendency towards esterification
arrange the following alcohols in the increasing order of tendency towards esterification
-
UP 0 DOWN 0 0 11

11 Answers
please give the logic behind it . I think my order is fine as in esterification , the attack of the alcohol on protonated acid is the rds , which depends on the steric factors , isn't C more sterically hinderd than A?
hi dude , check this out , the red mark indicates the O which attacks. the protonated acid. hope this helps. 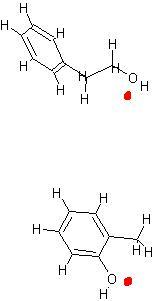
______________________________________________
______________________________________________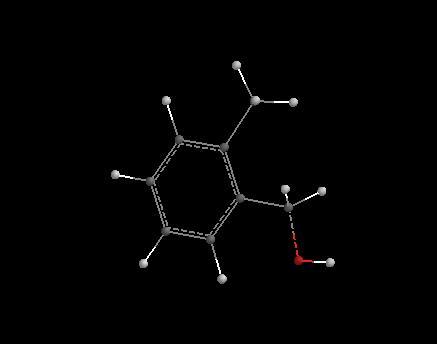
Now Decide....
i think it should be c>b>a (not sure)
bcoz c has most stable carbocation
yah...steric hindrance plays the role so the gp to be attached will have less free path to give the pdt..hence the order shud b
B<C<A correct me if im rong
even i think because of steric hinderance....but this is chemistry......may be there are other reasons which play an important role in deciding the order....lets think of it...