Lol no one? Okay here it is -:
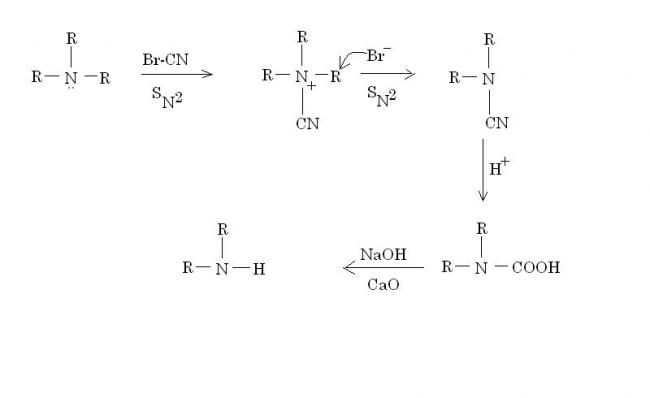
Br-CN is known as cyanogen bromide.
Br- vanishes as RBr(side product).
In what way can you convert (CH3)3N to (CH3)2NH?
Not a doubt. Try...and later I'll mention the direct method.
Lol no one? Okay here it is -:

Br-CN is known as cyanogen bromide.
Br- vanishes as RBr(side product).
if u dont mind can u tell the mechanism of hydrolysis of nitrile
am always kinda confused with that
secondly
tell the mechanism of the reagent NaOH/CaO
with carboxlic acid
i had always thought of hofmman bromamide degradation and borodine hunsdiecker rxn are the only way to reduce the number of carbon
I don't know the mechanism of the second, but Wiki suggests that :
"Such reactions proceed via the intermediacy of metal carboxylate complexes.
Decarboxylation of aryl carboxylates can generate the equivalent of the corresponding aryl anion, which in turn can undergo cross coupling reactions."
In my knowledge NaOH is simply used to spawn the sodium salt of the carboxylic acid, which is decarboxylated by CaO. NaOH may also play the role of a basic medium.
For the first, however, note that acidic hydrolysis yields the carboxylic acid while alkaline hydrolysis yields its salt. Mild hydrolysis yields the amide.
Step I : R-C≡N is first protonated to form R-C≡N+-H.
Step II : Water adds as a nucleophile at the electrophilic carbon center, causing electromeric shift and forming
R-C(H2O+)=N-H. Now a proton leaves to relieve O of its charge and attacks nitrogen.
Step III : We now have R-C(OH)=N+H2. Another molecule of water adds to C and another shift takes place, forming
R-C(OH)(H2O+)-NH2
Step IV : Another proton leaves to relieve the second O of its charge and attacks nitrogen, forming R-C(OH)2-N+H3.
Step V : Now protonated ammonia is a good leaving group, and it leaves. We have R-C(OH)2 left.
Step VI : The rest is automatic, as two -OH groups are too repulsed to stay on the same carbon, one forms a pi-bond with C and loses its proton. We get RCOOH.
For alkaline hydrolysis, these are the steps -:
Step I : Addition of OH- as nucleophile to C, causing electromeric shift. We obtain R-C(OH)=N-.
Step II : A proton adds to the nitrogen. We have R-C(OH)=NH.
Step III : OH loses its proton as it forms a pi bond with C, forming minus charge on nitrogen again, which is relieved by the proton OH lost. We now have RCONH2, or the amide.
Step IV : Hydroxyl ion adds as nucleophile again, while C=O becomes C-O-. Now we have RC(OH)(O-)NH2.
Step V : To re-establish the pi bond, NH2 leaves although reluctantly on the action of heat. We now have RCOOH and NH2-.
Step VI : To stabilise itself, NH2- abstracts a proton from RCOOH in a fast acid base reaction. Salt formation takes place thus. Then we have RCOO(Metal) + NH3.