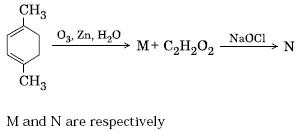i agree wth ur answer,sir.
10 Answers
It depends on the no of moles of the reactant.
If I assume that the added Ozone and NaOCl are in access..
then
M will be CH3COCH2CH2COCH3
if NaOCl is in excess
then N should be
NaOOCH2CH2COONa
CCl3COCH2CH2COCCl3 will be the intermediate.
yes nishant bhaiya i agree with ur ans...
the first one is reductive ozonolysis and the second one is iodoform in which the methyl keto will convert its iodoform....
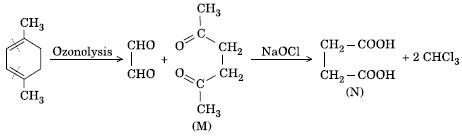
bhaiyya ur second one is wrong i think
n really sorry for the late reply :)
can u give the mech of the second one
subash you are right.. but for the reaction you are doing .. if there was a constrain to the amount of NaOCl then only one side would have undergone iodoform
what do you think?
no nishant is right . only on aicdification do we get the product given by Subhash