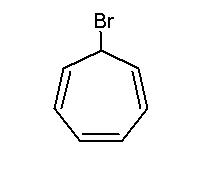
The answer is indeed (a). The intermediate obtained is the tropylium cation, a carbocation which is highly stable due to aromaticity.
Silver has a high affinity for halide ions. So it abstracts the bromide ion, irrespective of whether the resulting cation is stable or not (excluding the case of the benzene ring). A precipitate is formed (AgBr), which drives(forces) the reaction equilibrium forward to completion.