4> 2 >1 >5>3 ??
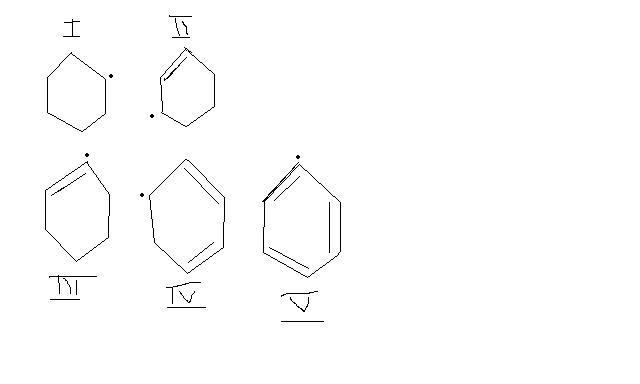
Arrange in order of increasing stability.
WITH REASONS..........
-
UP 0 DOWN 0 0 8

8 Answers
http://www.goiit.com/posts/list/organic-chemistry-arrange-in-order-of-increasing-stability-1031160.htm
That ques is asked by me there also but I have'nt got proper explanation........
ok , so i m wrong for 3 and 5
but see concept is that more the canonical forms , more stable it is . (try drawing dem )
And vinylic radical is unstable .
see , secondary carbocations are more stable than vinylic C+ .
similarly for radicals,
so , 1 > 3 and 5 .
and since 2 and 4 are allylic, 2 and 4 > 1 .
out of 4 and 2, 4 has extended conjugation so 4 > 2
@euracle: the correct explanation has been given there (i think it seems proper enough)
That is the correct answer euracle, and now I'll explain why -:
V - In this radical, benzene's aromaticity has been severely hindered. This radical has been stopped from resonating(it is a different matter if resonance were altogether impossible), and hence the loss of electron destabilises the substrate.
III : It is a similar case here, only instead of hi-fi aromaticity, it is only resonance which is being hindered here. Had the radical formed at an adjacent position, and not on the bond, it would have formed an allyl radical(which resonates). This type of radical is called a vinyl radical, and again is very unstable.
I : This radical is just a secondary radical. There are no extra stabilising factors. So this comes next.
II : This is a single allyl radical. Resonance imparts stability to it and makes it more inert.
IV : The most stable, this is a double allyl radical. It has the most resonance structures(canonical) and hence more contribution to its resonance hybrid and resonance energy. So it is the most stable of all.