1: reason is false
3. reason is false
4. both are true but reason dosent explain the assertion
2. same as 4
5: same as 4
1. Statement-I : Benzoic acid is a weaker acid than formic acid
Statement-II : Phenyl group when attached to carbonyl group becomes electron donating.
2. Statement-I : Pb4+ can be reduced rasily to Pb2+
Statement-II : Pb2+ is paramagnetic
3. Statement-I : HNO3 is stronger acid than HNO2.
Statement-II : In HNO3 there are two N – O bonds whereas in HNO2, there is only one N – O bond
4. Statement-I : Zn2+ is diamagnetic.
Statement-II : The electrons are lost from 4s – orbital to form Zn2+.
5. Statement-I : Nitration of anilline can be done by protecting – NH2 group through acetylation.
Statement-II : Acetylation of aniline results in the increase of electron density in the benzene ring.
-
UP 0 DOWN 0 0 10

10 Answers
1)both true
2)reason false
3)both true(electron withdrawing effect of two oxygen stabilises the conjugate base)
5)reason false
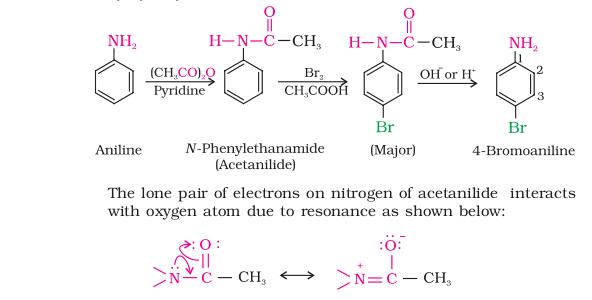

So acetylation of aniline electron density decreases on benzene ring ..
Pb belongs to grp 14 carbon grp..config ns2np2 if 2 electrons are lost from p the cation becomes dimagnetic...