aacc to me it must be none but a ns is acd i dont know why
IN W HICH OF D FOLLOWING COMPOUNDS CHIRAL CENTRE IS NOT AFFECTED BY HEATING?
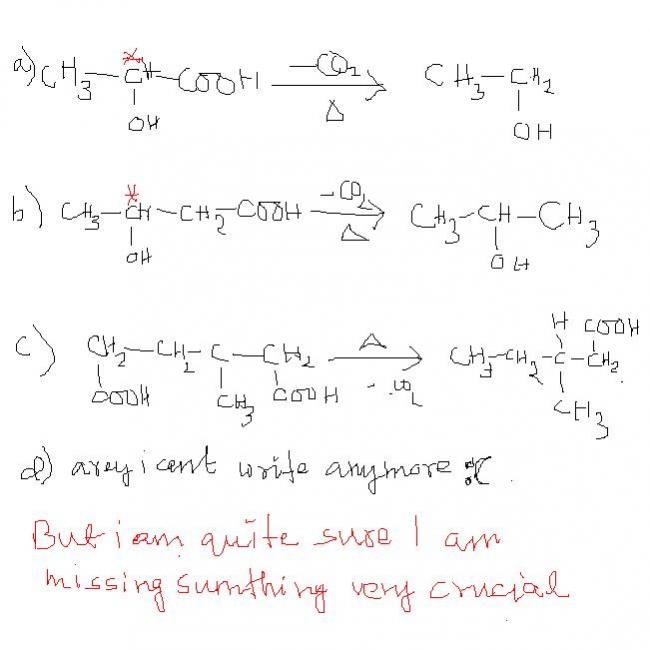
A CH3C(OH)COOH
BCH3C(OH)CH2COOH
C 2METHYL-BUTAN1,4-DIOIC ACID
D BETA KETO ACID
-
UP 0 DOWN 0 0 13

13 Answers
option c can be straight away ruled out. as on heating a dicarboxylic acid we get the according product.
i think answer should be d, as i see no possible efect o heating. { am i rite richa?}
baap re baap aaj mere saare answers galat. i know my brain as gone to hell. well srinath should see this thread
now i try to explain the answers as i know them............
that dioic acid wala, even though we get a product but that carbon with methyl wont be affected, so chiral centre would remain as it is.
ans is d)
the logic is unknown (rather not needed as far as jee questions r concerned)
a list where decarboxylation takes place on heating
a)beta keto acids
b)geminal carboxylic acids
Thats it!![1]
HEY SKY GIRL I VE GOT D ANS FOR 1 2 OH IS HYDROGEN BONDED WID COOH WID 5 N 6 MEMBER RING SO NO ALKENE
EVEN IF U WANNA DO DECARBOXYLATION A FIVE MEMBER RING
LL BE FORMED
ohk!!
thanx!!
i was thinking that only ... ki main kuchh miss kar rahi hoon ... kuchh bond ya kuchh...
now its seeming a'r8 :)
tx.