i'm not able to understand your doubt da. plz bolo .cele
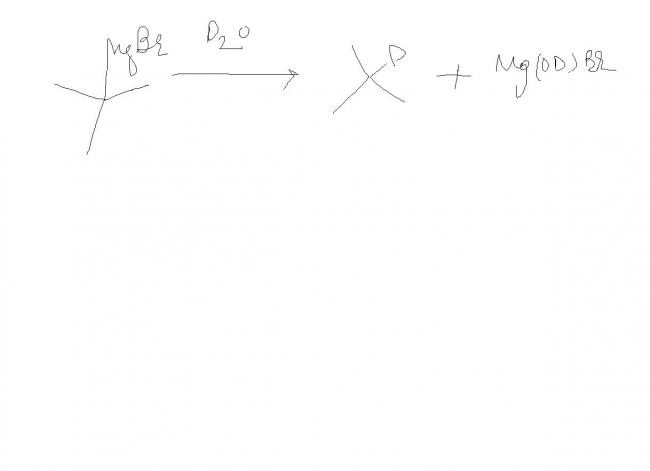
tri methyl methane magnesium bromide on adding heavy water gives what ?
ans gvn (CH3)3 -C-D
but why isnt it ( CD3)3-C-D ??????
-
UP 0 DOWN 0 0 20

20 Answers
no this is not due to steric hindrance...
i saw it today...
this is called beta-secondary isotone effect. [saw in pradeep's .. this came in CBSE-PMT once]
well the reason is: (CH3)3-C+ is more stable than (CD3)3C+
because of hyperconjugation of C-H bonds which can easily break ..
but C-D bond being stronger cant break easily so here hyperconjugation effct is lesss.
ok
now i thought the product wud react with more D20 to give ( CD3)3-C-D to maintain eq constant ratio for rxn
( CD3)3-C-D → (CH3)3 -C-D
no doesn't look like so .please tell . I'll post one thing is it what you've wanted ? just tell ok ???
leave it sri i think i had gone tooo far
i mixed up chemical knietics and thermodynamics with this organic rxn
the carbanion formed is very unstable reacts with D2O to form
(CH3)3CD
why D will replaced be H?
celestine bhai refer to NCERT bond dissociation energies of both D and H r given in the chapter "HYDROGEN" and u may confirm through there
well celestine what's your doubt . view this as a acid base reaction da. Then you'll be comfortable ??
so acc to ur post C-D bond stronger than C-H so rxn tends to favour the formation of ( CD3)3-C-D
hmm... now manipal.. y did u delete your post about C-H bond stronger than C-D one?
ppl will think m mad now... shouting at no one [3]
NO!!!!!!!!
C-D BOND IS STRONGER THAN C-H
REASON: MASS OF D > MASS OF H
NOW, FOR ANY BOND TO BREAK, THERE MUST BE SOME VIBRATION..
BUT VIBRATION REQUIRED INCREASES WITH INCREASE IN MASS.
AND GREATER THE VIBRATION REQD, GREATER THE BOND-DISSOCATION ENERGY.
SO..
ITS DIFFICULT TO BREAK C-D THAN C-H..
N.B :- THIS IS IMP!
i thought due to eq btw C-H and C-D in prescence of excess D2O , some H2O also formed ???
his occurs through SN1 mechanism as (CH3-) group is a bulky group and will pose steric hindrance
The R+ formed is unstable and rapidly undergoes nucleophilic attack
the whole CH3- group will remain intact and D will attack to give the req product!!!!!!!!!