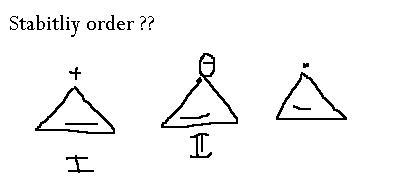
I > III > II?
10 Answers
Cyclopropane rings are highly strained because their bonds are packed at 60 degree angles. If they are attached to electron withdrawing groups, or if they gain a positive charge, they will gain a little stability. Thus the order of preference.
Carbocation is the most electron deficient, followed by radical followed by carbanion.
i thought we had to do this on aromatic stablity instead ......since it was attached to a aromatic passage...
Well thats how my friend explained it to me...I find this explanation easier lol
But yes, it should be done on basis of aromaticity, because a double bond is there...woh toh dekha hi nahi maine!
The reason comes from aromaticity in this case..
ok now what abt this ...arrange them in the increasing order of stability,..
Ph3C+ , tropylium , compound 1 given in abv question...
the attached image is of tropylium
triphenyl carbocation(9)>tropylium(7)> comp 1 above(3) resonating structures
tropolium > comp 1> triphenyl carbocation
tropylium...it doesnot lose aromaticity..
compound 1 > tropylium > Ph3C+
1014 1011 1 relative order of stability
Source : Peter Sykes