1)quote from wikipedia-
"If other steps are partially rate-determining, the effect of isotopic substitution will be masked."
since the slow step in aromatic substitution is formation of complex so the rate of both benzene and C6D6 is same.
Q1 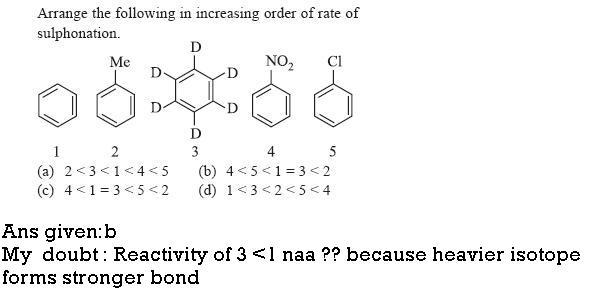
Q2 
Q3 Which of the following is true?
(a) O2– is a hard ion (b) O2+ is a hard ion
(c) O2– is liquid ion
(d) Both (b) and (c) are correct.
1)quote from wikipedia-
"If other steps are partially rate-determining, the effect of isotopic substitution will be masked."
since the slow step in aromatic substitution is formation of complex so the rate of both benzene and C6D6 is same.
Q1. if it was for ANY OTHER type of electrophilic substitution, then ans given would have been correct.
However, sulphonation is REVERSIBLE, so isotopic effect DOES come into picture, hence only for sulphonation 1 > 3...
read any standard book (i think given in morrison) for more explanation