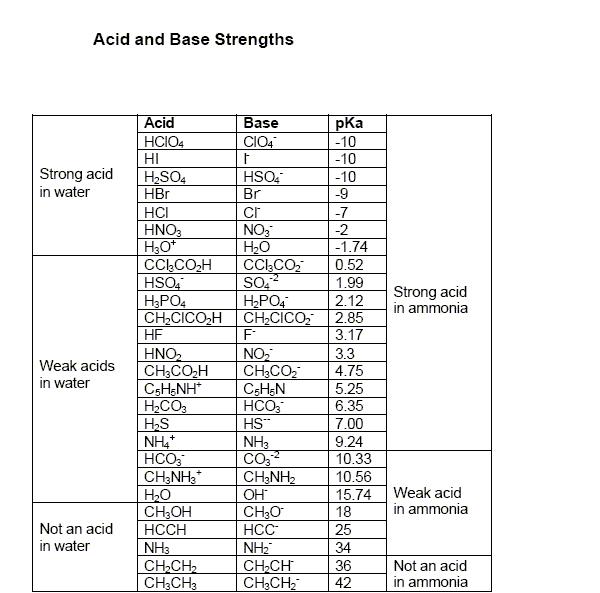
Q1. pKa value of H3PO4 is 2.15 as it is a weak acid. while HNO3 is a strong acid.. In nitration, the nitric acid acts as the base (accepts H+ thereby increasing the rate of formation of the nitronium ion) as it is weaker than H2SO4
But in presence of H3PO4 it will act as an acid i.e. donate H+ to form NO3- ion.. Now there is some amount of common ion effect so conc of NO3- will be reduced..
As NO3- can disproportionate (although quite little) to give NO2+ but due to common ion this will be further reduced to become negligible.
Hence NO
Q3. it is a reversible reaction. But I still think first step is rate determining step. Where did you read that?