 39
39In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent.
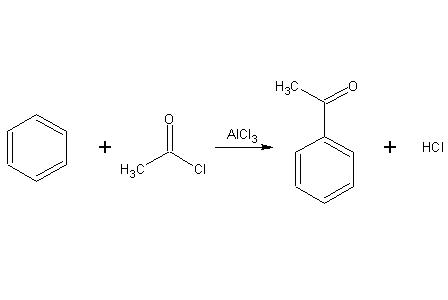
Because they form a strong electrophile when treated with some metal catalysts, acyl halides are commonly used as acylating agents. For example, Friedel-Crafts acylation uses acetyl chloride (ethanoyl chloride), CH3COCl, as the agent and aluminum chloride (AlCl3) as a catalyst to add an ethanoyl(acetyl) group to benzene:
 39
39The mechanism of this reaction is electrophilic substitution.
Acyl halides and anhydrides of carboxylic acids are also commonly used acylating agents to acylate amines to form amides or acylate alcohols to form esters. The amines and alcohols are nucleophiles; the mechanism is nucleophilic addition-elimination. Succinic acid is also commonly used in a specific type of acylation called succination. Oversuccination occurs when more than one succinate adds to a single compound. An industrial example of acylation in the synthesis of aspirin, in which salicylic acid is acylated by acetic anhydride.
 39
39Acetylation (ethanoylation) describes a reaction that introduces an acetyl functional group into an organic compound. Deacetylation is the removal of the acetyl group.
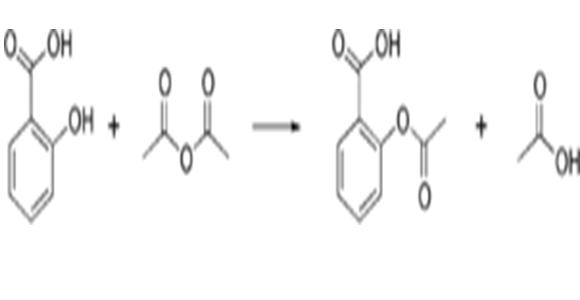
Moreover, it is that process of introducing an acetyl group (resulting in an acetoxy group) into a compound, specifically, the substitution of an acetyl group for an active hydrogen atom. A reaction involving the replacement of the hydrogen atom of a hydroxyl group with an acetyl group (CH3 CO) yields a specific ester, the acetate. Acetic anhydride is commonly used as an acetylating agent reacting with free hydroxyl groups. For example, it is used in the synthesis of Aspirin.
 39
39N-alpha-terminal Acetylation
Acetylation of the N-terminal alpha-amine of proteins is a widespread modification in eukaryotes. 40-50% of yeast proteins, and 80-90% of human proteins are modified in this manner, and the pattern of modification is found to be conserved throughout evolution. The modification is performed by N-alpha-acetyltransferases (NATs), a sub-family of the GNAT superfamily of acetyltransferases, which also include histone acetyl transferases. The GNATs transfer the acetylgroup from acetyl-coenzyme A to the amine group. The NATs have been most extensively studied in yeast. Here three NAT complexes, NatA/B/C, have been found to perform most N-alpha-terminal acetylations. They have sequence specificity for their substrates, and it is believed that they are associated with the ribosome, where they acetylate nascent polypeptides co-translationally.
In humans, the human NatA and NatB complexes have been identified and characterized. Subunits of the human NatA complex have been coupled to cancer-related processes such as hypoxia-response and the beta-catenin pathway. It has been found to be over-expressed in papillary thyroid carcinoma and neuroblastoma. The human NatB complex have been coupled to cell cycle. The hNat3 subunit of the hNatB complex have been found overexpressed in some forms of cancer.
Despite being such a conserved and widespread modification, little is known about the biological role of N-alpha-terminal acetylation. Proteins such as actin and tropomyosin have been found to be dependent of NatB acetylation to form proper actin filaments. This is yet only an example of the potential importance of this modification

