 39
39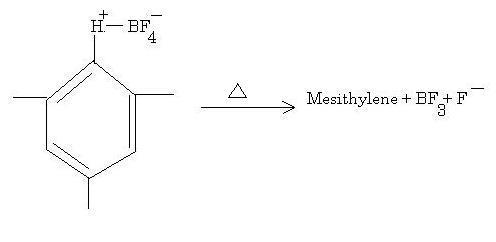
This is an EAS reaction where a proton is the electrophile. Normally this reagent is used in conjunction with CO to form benzaldehydes. BF3 is used as a Lewis acid to abstract the F- ion to form an electrophile complex much in the same way AlCl3 takes up Cl- in FC reactions.
The substrate on the left is your bright yellow solid, ionic in nature due to the full charges present, thus conducting electricity when molten.
It is a strong support for the EAS mechanism because of the evidence of the electrophile complex which attaches to benzene, which when heated regenerates the original compound.
Hardly see any of these sort of questions coming in JEE, as per this year's paper...you'd be better off learning your theory from M & B like you are now and solving standard questions. This could possibly be a paragraph question though..
 1
1Q1>Find Z:
benzene---Ac2O/AlCl3-->X---Cl2/AlCl3--> Y---Zn-Hg/Hcl-->Z
Q2>Aniline---Ac2O/Pyridine-->X---Cl2/AlCl3--> Y--water-->Z
In the second question how to use pyridine ???
 39
39Q1. 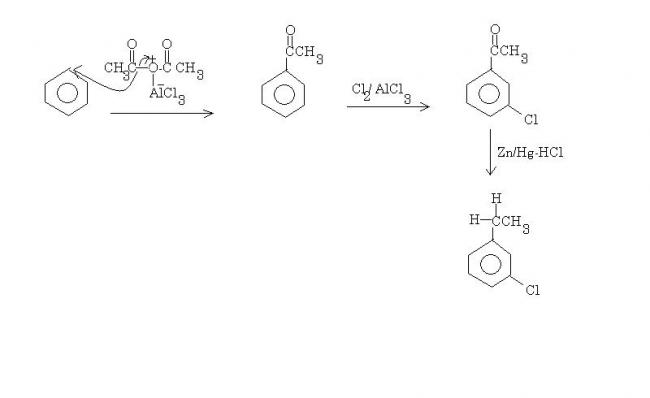
Q2. Pyridine is just a catalyst, it performs the same function as do the Lewis acids(due to the nitrogen group's electron withdrawing nature here).
X is acetanilide. Y is m-chloroacetanilide. Hydrolysis removes the acetyl group, and the end product is m-chloroaniline.
 1
1Thanks Pritish!!
Q1 is right
but
Q2> answer given ins mixture of ortho and para chloro-aniline
ok take these Qs:
Q3 the product of reaction:
Dimethyl phenyl phosphate---HNO3--->??
 39
39That's not possible...acetanilide group is electron withdrawing.
And is the nomenclature right in Q3?? Only superacids or superbases are able to ionize benzene..lol. I presume it's a PO3 or some group attached to benzene. In that case it could become di methyl nitrobenzene.
 1
1exactly pritish it is EWG...so ans is wwrong kya??
ya nomenclature is right

