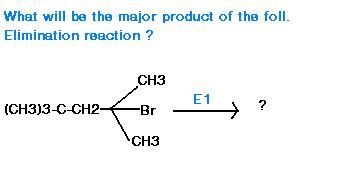
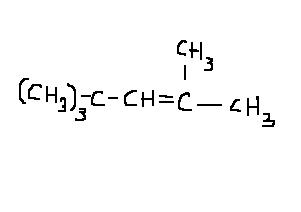avik havent u seen application of hoffmann rule ever b4 in elimination ???
[ See solomons and fryhle book ...i saw it there ]
34 Answers
Got it .....SOurce : Google !!!!
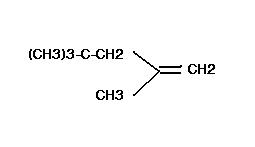
Hofmann's Rule implies that steric effects have the greatest influence on the outcome of the Hofmann or similar eliminations. The loss of the β-hydrogen occurs preferably from the most unhindered (least substituted) position [-CH3 > -CH2-R > -CH(R2)]. The product alkene with fewer substitutents will predominate.
Ester Pyrolysis also obeys this preference, and the Hofmann Rule is generally followed whenever a reaction passes through a cyclic transition state.
Hofmann's Rule is valid for all intramolecular eliminations and for the Hofmann Elimination. Most bimolecular eliminations will follow Saytzeff's Rule.
so when should we follow the Saytzeff's rule and when should we follow the Hoffman's rule in E1 mechanism??
Hmm quite possibly so, I didn't think of it that way. Where the base finds it easier to abstract a proton...
The answer given by avik is indeed correct, hyperconjugation is ruled out as the main carbon in the tert-butyl group has no hydrogens. So it has to be steric hindrance.
[11] !!! But......wat to say, kuch samajh nahi aa raha..
Matlab ab everywhere during abstraction of a proton, v need 2 consider hoffman as well ?? I have applied hoffman e. only in amines n nowhere else till date...
Steric hindrance toh har 30 Carbocation ke saath hoga...
Lekin, Aaj tak jo kai saare saytzeff products banaate rahein hain after a 30 C+....un sabka kya karein ???
This has been the 1st Qn ever...nopes, the 1st with this sort of answer ever...
is the base used mentioned in the question??
if it is a bulky base (even if CH3CH2CH2O- )
we will go for hoffmann product
wait.. you ought to consider hoffman's probability everytime you are about to do elimination. And it's not just case with quartenary amines that you will do hoffman elimination. There are 2-3 other cases in which you will have to consider hoffman.
so u say dat zaitsev rule has to compulsory be obeyed by hydrocarbons???i doubt dat ..dude if u use a tert butoxide ion as a base for the question posted in this topic ....the major product has to be the least substituted alkene..due to steric hindrance ..i.e violation of zaitsev rule ...wich is also called as hoffmann rule ...[ source: solomons 8th edition ]
Actually my prof said that ...hoffmann elimination is related to quarternary ammonium salts ...where the alkyl grp gets eliminated ....and over there least substituted alkene is the major product..wich is the hoffmann's rule ....
so i think since steric hindrance causes the violation of saitzev's rule ... and produces least substituted alkene ...it is also follow hoffmann rule ...
heyy..i'm still confused.
In the product i've shown, the hydrogen is abstracted from the carbon which is not very sterically hindered. It is attached to 3° carbon but it is not 3° itself. It just has Hydrogen on it.
So do we still consider the Hoffman Rule??
The carbon beta to the carbocation will have a sterically hindered group in tert butyl(branching), which causes the base to abstract proton elsewhere. We consider only upto the beta carbon.
Reopening the debate here.....
First of all...you know what Avik? This reaction won't follow E1. It will follow E2(atleast try to)...lol.
For E1 : 3° > 2° > 1° (Carbocation stability order)
For E2 : 3° > 2° > 1° (Hyperconjugation of transition state)
And if E2 is going to be followed, you might as well have Hoffman trying to pester it.
Why most of us were confused at first is because Avik has given the substrate to strictly follow E1...which is not the case because E2 occurs by a transition state(partial charge more stable than full charge) and is more favourable(even though both follow the same stability order).
The Zaitsev rule applies to E2(only bimolecular eliminations follow it). E1 is judged by the more stable carbocation.
And now that Zaitsev can be followed...we find that the steric conditions of the substrate take full toll. Hence the Hoffman rule dominates.
Yes, now it fits..!! E2 hoga, agreed, and so will the Hoffmann eliminated product follow.
So, it turns out tht the Qn was indeed wrong ;) .
@Pritish... How come u remembered tracking dwn a 5 & a half month old Qn ?!! Commendable!
Just happened to be looking at some of my old posts to see if any threads were left unanswered...and found this :P tab mere dimaag mein aaya..why should E1 specifically take place at all? And so here we are.
Arey he has predicted the product with the most stable carbocation only...except the base abstracts a different beta-hydrogen then we expected! Which proton a base abstracts depends on the proton's relative acidity.
prongs' answer should be it...tert-butyl shift can also happen but that will cause a primary carbocation to be formed. The major product should be as prongs has shown it.
agreed!....also, there is no need of a tert-butyl shift because we already have a stable tertiary carbocation !
There would be two other products depending on which beta hydrogen is abstracted by the base..
Possibly the tert-butyl group's hyperconjugative effects weaken the acidity of the proton present. The more acidic proton is favoured to be abstracted by the base.
but answer should be as told by Prongs if E1 reaction is taking place. Here it can't be even contradicted by stating steric hindrance effect and I can't see any other problem in elimination.. still thinking.
yeah....in E1 mechanism, the intermediate should be the most stable carbocation.
why arent u guys looking at the steric effect ??? hoffmann elimination!!!
carbocation will be 3o,
but the Hydrogen that will be abstracted by the base will be the one that can be abstracted with relatively more ease!!
Coz it says E1...
E1CB occurs only when the leaving group is poor :\
sry sry ..i completely messed up by using dat word ...but it shud be hoffmann elimination na ?