The Grignard reagent attacks at the least substituted side of the carbon-oxygen bonds, if there is one. In this case, one carbon has 2 hydrogens and the other has 1, so the R group attacks the carbon with 2 hydrogens, breaking the bond with oxygen which is then protonated by the acidic solution. leaving a secondary alcohol and a concatenated carbon chain. The R group can be alkyl or aryl.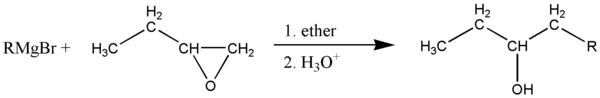
copied from wiki