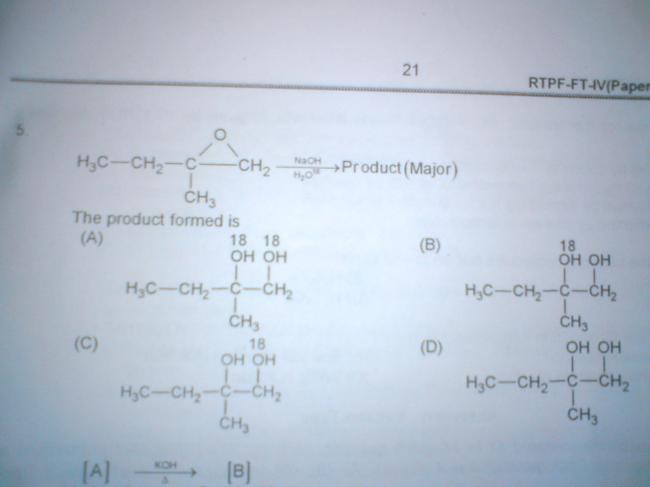
ya but why does NaOH have more preference to act as nucleophile when water is solvent and is present in larger qty?
........................................................................
.......................................................................
no the answer is not C
-
UP 0 DOWN 0 0 12

12 Answers
ya but nucleophilicity of water molecule is very low it cant break epoxide ring if there would ve been a better nu as solvent then the major product would ve been changed
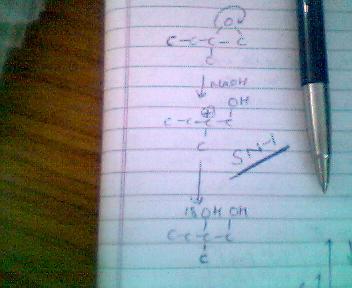
therefore the answer is B according to the SN1 mechanism in case of a 3° carbocation formation!!!!!!!!!
cheers!!!!!!!!
@aki
what you've shown takes place in acidic medium,in basic medium carbocation is formed at least sterically hindered position
d hi answer hai... to ye aki B kaise laya...
C+ banane ke baad OH- frm NaOH attack karega... na ki H2O as she had expalined in post5 ..
haan bhaiya pink kar dijiye... :P