hhhhmm same doubt here .........[131]
Why LiAlH4 can't reduce the double bond with conjugation with carbonyl group but it can reduce when phenyl group is attached at β carbon ??????
-
UP 0 DOWN 0 0 7

7 Answers
Even our sir mentioned this...this is my doubt too. I wonder why it happens.
hey i m also having doubts abt it ,
my sir told that LiAlH4 and NaBH4 cant reduce double bonds.
WHy??
and pls tell me all such exceptions!!
Lithium aluminum hydride
LiAlH4 is a strong, unselective reducing agent for polar double bonds, most easily thought of as a source of H-. It will reduce aldehydes, ketones, esters, carboxylic acid chlorides, carboxylic acids and even carboxylate salts to alcohols. Amides and nitriles are reduced to amines. In each case the partially negative hydrogen reacts with the partially positive carbon of the substrate. It can also be used to reduce nitro groups and even as a nucleophile to displace halide from an sp3 carbon or open an epoxide.
Why does it work? Remember that aluminum is a metal with a low electronegativity; thus, the Al-H bond is strongly polarized with Al positive and H negative. The abnormal polarization (and oxidation state of -1) for hydrogen, which is normally positive, results in a high reactivity, especially with atoms that can accept electrons (be reduced), allowing the hydrogen to become positive again (normal oxidation state of +1).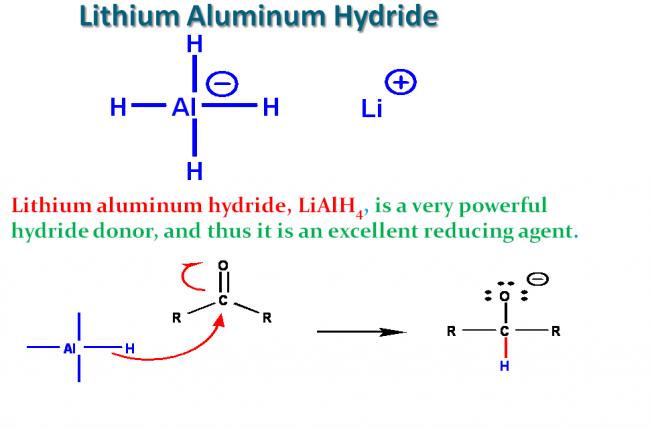
Precautions: It reacts with other positive centers as well, especially any slightly acidic hydrogens, like those of alcohols, water, carboxylic acids or alkynes to produce hydrogen gas, which is highly flammable and can readily explode (e.g. the Hindenberg). LiAlH4 requires anhydrous conditions for the reaction and usually an excess of the reagent (to soak up any water that was missed in the glassware or reagents); the most common solvent for the reactions is diethyl ether. "Workup" at the end of the reaction is usually done by careful addition of aqueous acid (remember the flammable hydrogen gas and ether), followed by extraction of the organic products from the water-soluble salts.
To make LiAlH4 less reactive and more selective, the hydride is made more hindered, e.g., in the compound LiAl(OtBu)3H. It can reduce acid chlorides (and some esters) quickly, but is slow to react with aldehydes; therefore LiAl(OtBu)3H provides a convenient way to synthesize aldehydes by reduction of acid chlorides, something that can't be done with LiAlH4 or NaBH4.
Sodium borohydride
NaBH4 is less reactive than LiAlH4 but is otherwise similar. It is only powerful enough to reduce aldehydes, ketones and acid chlorides to alcohols: esters, amides, acids and nitriles are largely untouched. It can also behave as a nucleophile toward halides and epoxides. It is also convenient that, although LiAlH4 is strong enough to reduce the C=C of a conjugated carbonyl compound, NaBH4 is not; thus the carbonyl group can be reduced without the alkene.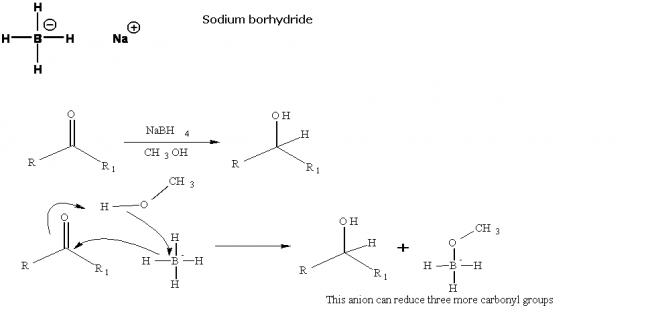
Precautions: NaBH4 is unreactive enough that the reductions can be done in alcohol solution, or even water (as long as they don't take too long); this can be advantageous for polar compounds which can be pretty insoluble in ether. Hydrolysis with acid and water followed by extraction is used to isolate the product (hydrogen gas is produced).
Thank you sir for the explanation , but one more request ...
pls can u post the structure of LiAl(OtBu)3H
sir my doubt is tht............... why the double bond is reduced when phenyl group is attached to the β carbon
i.e explain WITH MECHANISM how LAH can convert C6H5--CH=CH---CHO to C6H5--CH2--CH2---OH
hey forum expert..you have just copeid down the nswer from wikipedia...
without answering any of the original questions
i wonder how you made it to iit