39
39Badiya sawaal. First of all a list of reactions which we cannot use :
1. Carbonyl reductions(W-K, Clemmensen, etc)
2. Mozingo protection(addition of alcohol)
3. Ester hydrolysis(because nucleophilic addn can happen at the solitary ketone group too, and esters are less reactive to Nu addition than are ketones)
4. Normal reductions(LiAlH4, NaBH4, etc)
 11
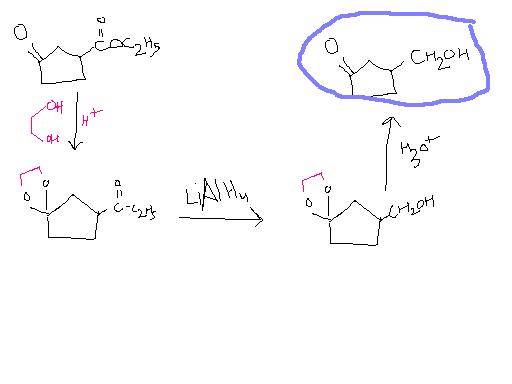
11@Pritish, LiAlH4 can be used , but not NaBH4
 1
1@ tushar LiAlH4 will reduce ketone
pritish 2 is the only way to protect the carbonyl group , we can form cyclic acetals that are stable
 39
39Yes sure. And then you'll reduce the ketone as well. And when you try to oxidise it back, -CH2OH ko kaun bachayega?
Ok if you say so RPF...seems to be no other method.
All reactions we could possibly use are based on nucleophilic addition or acid-catalysed enolisation.
 39
39You know what's wrong with this method?
-OH can leave and ring can expand...
 1
1well wat tushar has wriiten is correct
this is wat is given in solomon
@pritish
ur argument is also a valid one
but that will take place via SN1 mechanism so requires a great free energy barier to cross
so that will be a minor product (may be that y solomon has given yield to be 98%)
 39
39Well if Solomons says the method is fine I have no issues.