Q3 Gilman reagent, for 1,4-addition to alpha-beta unsaturated ketone ((CH3)2CuLi).
Q1. RLi is most reactive due to polarising power of lithium. Negative charge on R is maximum in this type of organometallic reagent.
In Q4, since there is no steric hindrance in the substrate, grignard reagent adds to the more polar C=O bond (1,2-addition). Hydrolysis of this gives alcohol in place of =O and the double bond is also hydrated into alcohol(as per Markownikov addition).
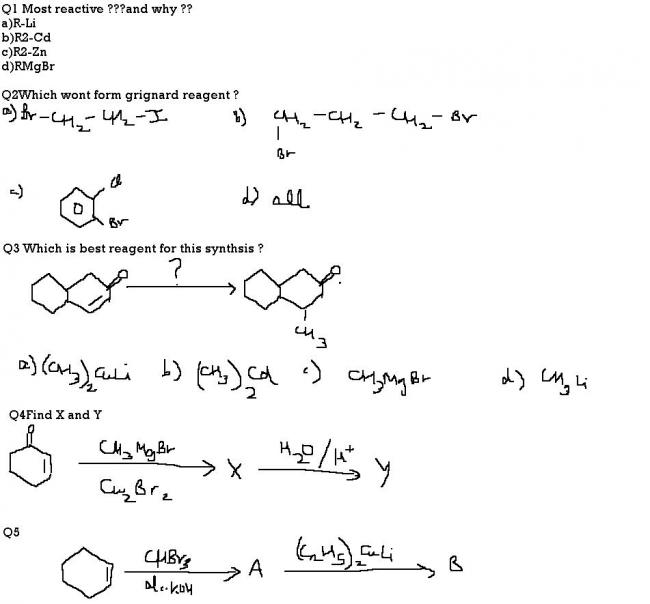
In Q5, dibromocarbene is formed by alpha elimination and it adds to alkene to form a dibromocyclopropane(base of the triangle is where the alkene is, and on top most vertex two bromine atoms are present). SInce Gilman reagent is an alkylating reagent when it comes to halides, it will remove the two bromine atoms and replace them with ethyl groups.