& yes the cmpnd is planar
1 more thing being planar or nt has nthing to do with the Q
conjugation means that huckel rule is followed? kis ne kaha?
wait I'll give a good example.
& yes the cmpnd is planar
1 more thing being planar or nt has nthing to do with the Q
kaha mila ki yeh cmpound is planar?
bolo . pleasee. come on dude . kaise ?
wait a min. dude
its an iit Q
how the hell is it nt planar??
evry ring is a conjugated system
conjugation means dat huckle rule is followed
if huckle rule is followed then cmpound is planar
i thnk i was rong wth huckle rule post
but then u prove y its not planar then i will share ma views
If it were planar . then there will be three cyclobutadiene parts in the same molecule , ???????? so they prefer to have non-planarity than this , as each benzene molecule part is sepaprately stable. y bring in instability when u can avoid it.????????
which huckel rule are u talking of ? (4n+2) no ? then the above compound is a very good example. I'm having difficulty trying to explain. will come up with one. give me some amopunt of time.
Yup . got it. what do you think is this compound planar?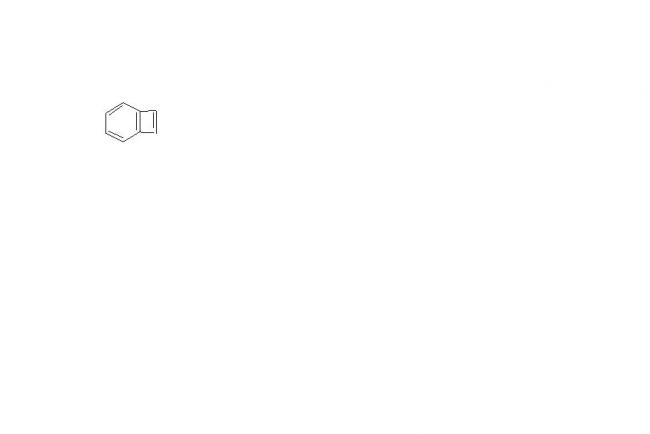 forget Huckel rule for a mo.
forget Huckel rule for a mo.
i still think it is planar so u better try to give the reason dat its not
& dont try to apply stearic effect here
are baba . where steric effect. I've never mentioned that word.
go only with anti-aromaticity and aromaticity only.
don't bring inconfusion here . U'll land up in more trouble.better stick to one concept.
well brght stearic effect in between as the only reason for me for it to b non planar is stearic effect dats y i am asking ur reasoning
Sorry guys..
This is planar
Don't ask me reason
as this is chemistry
But i'm posting an image
(don't ask me how i'll be able to do this in xam though as thats the reason why i always get less in chem)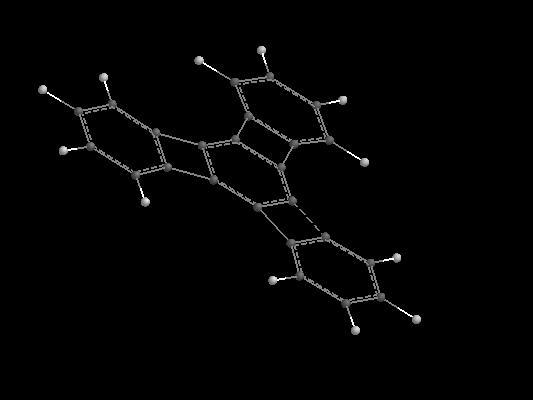
Thanks Abhishek .
There's something wrong with my reasoning .
sorry guys . my mistake.
it will be A.
my reason is a very useless one though ...
A is very symmetrical from all sides...
i am sorry to myself n others for such a stupid reason.. (but its chemistry!!!!!)
Looks like a UFO to me! So for easy storage, aliens will reduce all rings to as small a size as possible
P.S:
No hard feelings pls!
@ramkumar
only 3H2 are provided so only one ring can be reduced
I thought so because in A , all C are similar and they don't have any H and also i thought there will not be much conjugation...
nt a intelligent reason varun
hint * antiaromatic compounds provide high unstability
sorry .. i cldn see any ring over there... i was taught lik dis only..
i was taught lik dis only..
but plz do give the correct reason.. am curious... its a nice question b/w.
well sky if u visualize more clearly u will find dat we have 3 cyclobutadiene rings in the cmpnd
which r antiaromatic so by reducing da middle ring we will be able to get rid of them too
& yes A is symmetric from all sides bt then u cant proceed any frthr frm it
bt dis was a MCQ so u get full marks :D