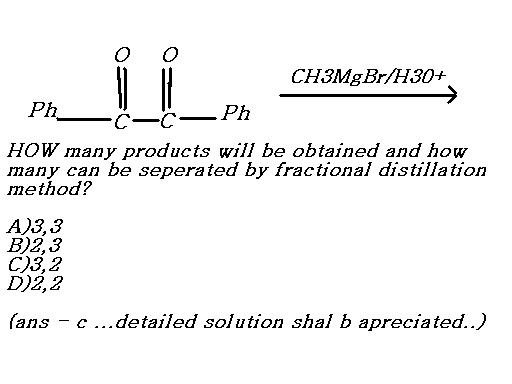
39
39Easy....there are three ways this reaction can proceed.
I. The Grignard attacks the left C=O and transforms it into alcohol(with methyl attacking C).
II. GR attacks right C=O, but overall the same product forms.
III. GR attacks both C=O and hydrolysis yields a dihydric alcohol.
I and II cannot be separated by fractional distillation as they are basically the same. III can be separated from I/II.
So there are overall 3 products, two of which can be fractionally distilled and separated.
 1
1yaar but 2 products are xactly the same
they will not be called diffrent
2 products are formed here
 1
1and maybe the third coz of Pinacol Pinacolone
 39
39Pinacol-pinacolone ke liye toh moderately acidic medium chaiye...whereas Grignard hydrolysis lightly acidic medium se bhi chal jaayega. Also you need to supply energy for the rearrangement and cation formation. Isliye I don't think pinacol reaction hoga(no heat given).
Nice thinking though Akshay...not everyone thinks that far ahead!
 1
1@ Pritish
I know Pinacol requires acidic medium but when we know the answer our mind tends to run in all the ways to reach it