look at dfferent in K and TsCl...the latter is very much bulkier! so attack is from opposite side
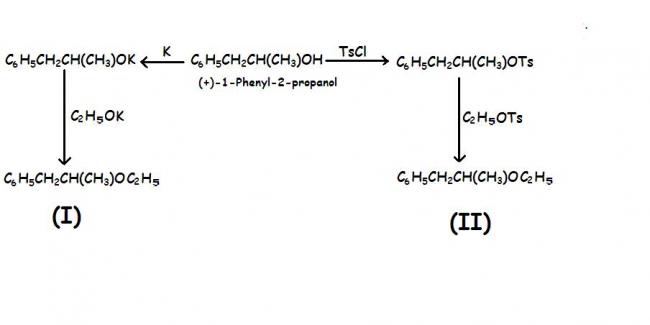
(I)→ Ethyl 1-phenyl-2-propyl ether α=+19.84°
(II)→Ethyl 1-phenyl-2-propyl ether α=--19.90°
a) account for the fact that the ethers obtained by two routes have opposite but equal optical rotations?
b) Does it matter what the optical purity of the strting alcohol is?
c) What is the fundamental significance of this finding??
oops..although i promised not to post nemore optical this too contains optical..[3][3]
-
UP 0 DOWN 0 0 9

9 Answers
No it's not about being bulky....the OTs group, or tosyl group, is a very good leaving group(as are methyl sulfonates, triflates and acetates). Even though the carbon is secondary, I guess the %age of SN2 would be much higher....this is an indirect method to prepare ethers.
I suppose the left side method is a poorer way to synthesise ethers as elimination competes. So the %age of SN2 is less? I can't say its an SN1 reaction, as Williamson synthesis uses SN2.
In Sn2, theres only the backside attack so u get only R or S, not both.
Now in these 2 shown reactions, they follow Sn2. But the product of one reaction is R and the other is S. meaning that in one reaction the "backside" was different from that of the other reaction. Why? How can you predict it???
The absolute configurations say NOTHING about the inversion of the molecule. I have explained this to subho...inversion of absolute configuration is merely a consequence of the fact that both the incoming nucleophile and the leaving group have the same priority.
Here's a little information : http://blog.para-gen.com/2008/06/41/williamson-ether-synthesis.html
It says Williamson ether synthesis can take place via SN1. So the best guess is that the potassium-induced synthesis is via SN1 while the tosylate synthesis is obviously SN2.
Maybe this has something to do with the fact that potassium has lesser polarising power than does sodium? Polarisability does affect SN2...
Now if our working hypothesis is correct, the optical purity of the alcohol does not matter, as in SN1 whatever specific rotation is achieved the opposite will be achieved in SN2.
:) I can't really say that I'm correct without confirmation here...but thanks all the same.
And also the fundamental significance of this finding is that Mr.Subhomoy Bakshi has been picking our brains since the last few days!
yes yes! i agree. and hmm, he keeps promising that no more optical, but somehow strangely, every question ends up coming back to it!! :P