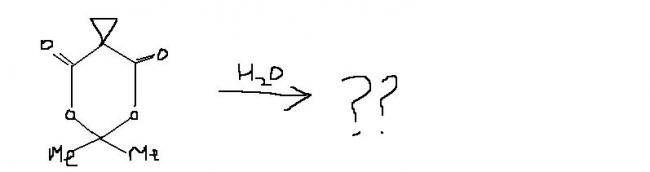
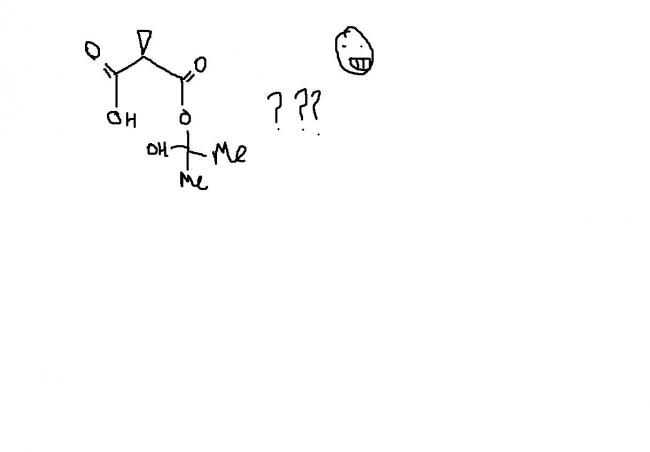
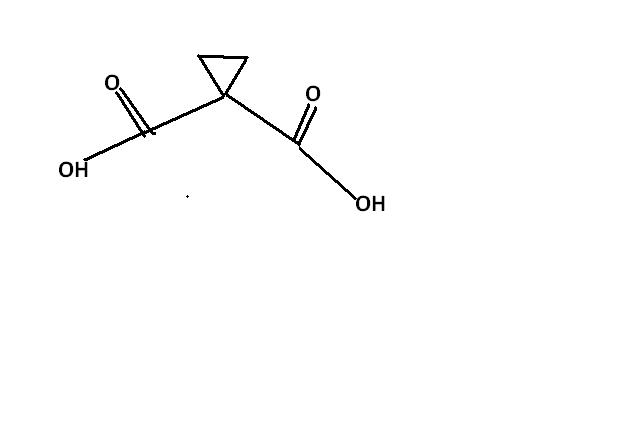
nops.....[2]
16 Answers
as far as i know that the hydrolysis reaction can be observed only if we add an acid,atleast to a small extent.
guys , this one i think , is an extended version of ester hydrolysis. the process continues becoz of hindered cyclopropane group attached. i'm first posting the normal ester hydrolysis and then what extra takes place here.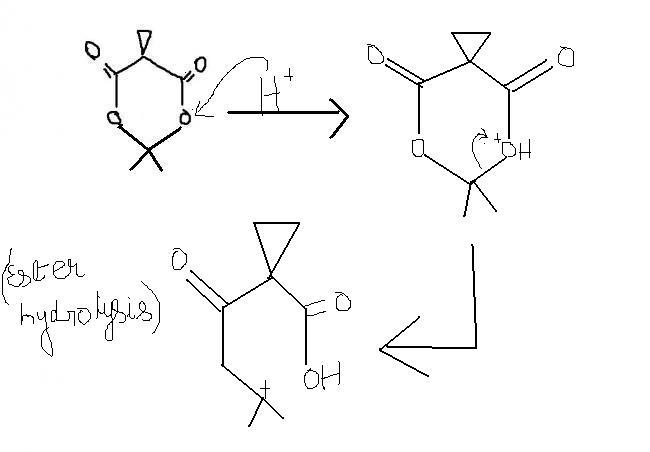
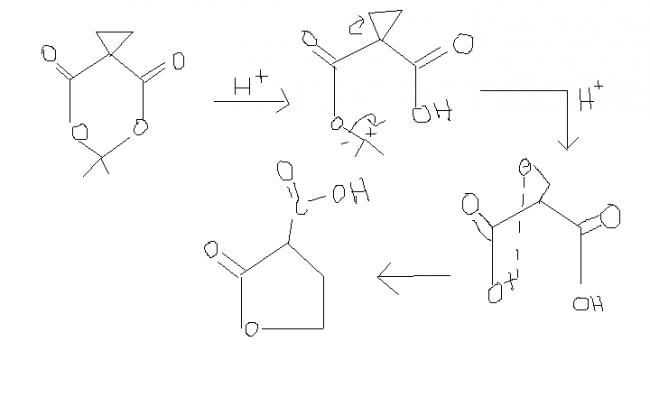
problems u may hv :
a) in the presence of acidic medium i.e. H+ , the cyclopropane ring splits.
b) +ve charge on O is unstable only when ders a less electronegative atom present near it, wid a possibility of transfer of +ve charge to less electronegative atom. here +ve charge on O can't be delocalised. thus, stability factor doesn't come into account.
xyz , then a carbocation will be left behind , with O on one side and a sterically hindered group on the other , both of which can't attain this +ve charge.
moreover, if dat way process continues, then a minor product is formed.
Mujhe second step samajh mein nahi aaya...the carbocation leaves or what? can someone please explain?
I've finally got the correct mechanism for this. After 2 hours of brainstorming with Ankur last night, we found it out.
Mujhe pehle se hi lag raha tha ki aieeee ke mechanism mein gadbad hai, even though he gets the right answer in the end, which is quite miraculous. What aroused my suspicions -:
I - That's not how cyclopropane cleaves due to a proton. Cyclopropanes are unique because they have a partial double bond character, and electrophilic reagents add to them in a Markownikov fashion. This opens up the ring.
II - That's not how ester hydrolysis takes place. The alkyl part can't just leave like that.
PS : Ask Ankur to allow me to post the mech. He doesn't think any of you will understand(the new comers) :P