Have a look.
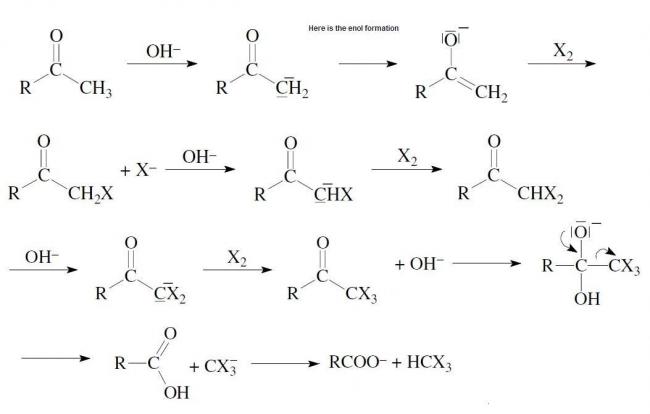
why 3,3dimethylbutan2one does not give iodoform test?
bas ravi tune kar diya mazak faaltu main.......
joke mat maar jawab de........
the compound is very stable there fore it doesn't give the iodoform test
I didn't said U r wrong.
I just wanted to show that there is enolate ion formation in the mechanism....
For the question u gave i'm not much convinced by the fact that due to enol formation poses a problem for reaction cause enolate ion are always in equilibrium with keto group and their concentration is larger in solution only when there is β diketones or any carbonyl compound in which there is resonance stabilisation or intramolecular H-bonding.
So I also think the main factor should be Stearic hindrance.....
There is a transition of Carbonyl group and the enolate ion at every stage....
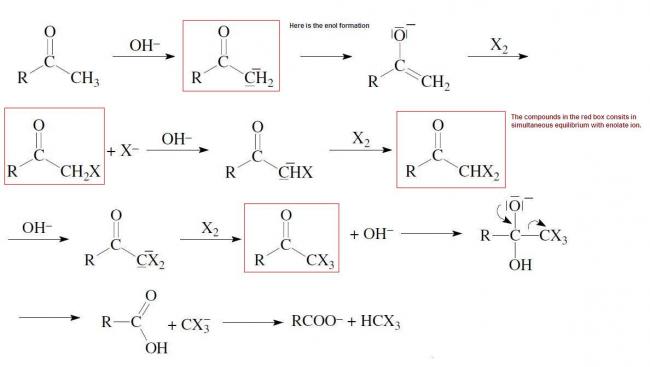
So the red boxes compounds in the diagram are always in equilibrium with enolate ion.
So u can't deny the fact that there is enolate ion in the reaction.
when enol intermediate form it favours in more substituted alkene & steric effect
I agree with rahul and there is enol formation during Iodoform reaction.
If u require i can post the whole mechanism.......
even i think so rahul u confirm d thing there is no enol formation
however i support u in stearic crowding reason
tell me if i m wroong
why ?????????????????????????? rahul i didn't get your point