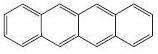
For planarity, check the hybridisation of all carbon atoms and convince yourself that they all are sp2. Second, it must satisfy Huckel's rule, i.e., must have (4n+2)Ï€ electrons. Since, the compound has 9 pi bonds, 18 pi electrons, which is of the form (4n+2). This will ensure, that the delocalisation is complete and throughout the ring, ensuring that the compound is aromatic. [1]
You could make a general thumb rule that if any aromatic ring is fused with another aromatic ring, then the resultant ring is also aromatic.