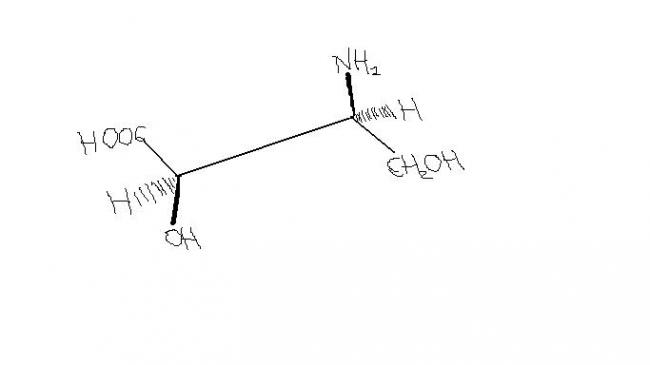
39
391. The boiling point increases as dipole moment increases because intermolecular dipole-dipole interactions/forces add to the already present London dispersion forces. Cis isomers usually have higher boiling point because of this.
However, for melting points, intermolecular forces aren't the only deciding factor. The packing of the molecule also plays an important role.
For a thorough explanation, see this page : http://www.chemguide.co.uk/basicorg/isomerism/geometric.html
2. Try to imagine it this way. You have two open palms. On the left palm you place the structure of the left compound the way you see it on this page. On the right palm you place the structure of the right-side compound as seen on the page. Now if you try to place one palm over the other, will the two structures super-impose? The answer is no.
Thus these two are enantiomers.
3. The pyramidal inversion effect, or umbrella effect as you call it, is a spontaneous process at room temperature which operates in amines having pyramidal geometry. The simplest example is ammonia. The molecule flips inside out around nitrogen's lone pair. Since this interconversion is so rapid(the energy barrier is very small and hence the process is spontaneous), acting like a conformational change, nitrogen is prevented from obtaining chirality.
However if the nitrogen is strained (being a bridgehead atom, for example), it becomes a chiral center and it cannot interconvert.
4. Google for more about this...don't remember.
5. My guess : The left side carbon is D and the right side carbon is L, using the C-O-R-N rule.
 1
12.ok,i understand that the two structures will not superimpose.then,
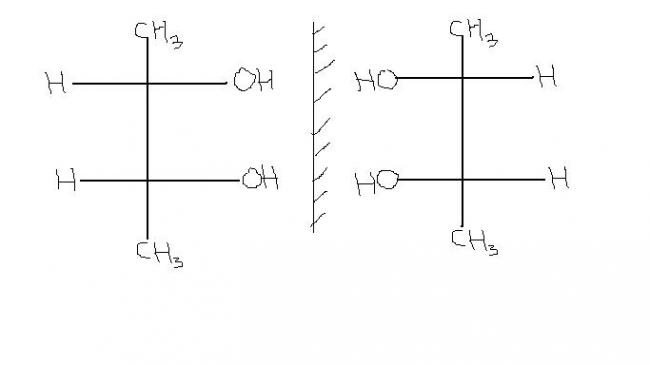
why are the above compounds superimposable mirror images?
if we place one mirror image on other,they will not superimpose.....i mean the OH grp will on H and H will fall on OH.
 39
39You're right, they're not superimposable mirror images. Who said they are?
 1
1these are superimposable....bcz acually the molecule extends in 3-D but when we convert it into 2-D (fischer projection) , then to check the superimposability, we can rotate it in the same plane..... and if u rotate the 2nd structure by 180° then it will get superimposed on the 1st structure....
while in ur 1st question, they wont superimpose, no matter how much u rotate it in the same plane....
 23
23@ pritish amandeep is right , they are superposable, rotate the mirror image by 180o , u get the exact earlier molecule
 11
11#3, the molecules uploaded in this post are superposable on each other , because when you rotate the mirror image by 180°, you gonna get the same image.
@ shardapanda, your earlier molecule of Pentan-2,3-diol is not superposable because even if
you rotate the mirror image by 180°, then methyl grp will fall on ethyl grp on methyl. And the
condition for superposability is that, same atoms or molecules should fall upon the same ones.
Only -OH grp falling on each other can't make that mirror image molecule superposable on its
earlier molecule.
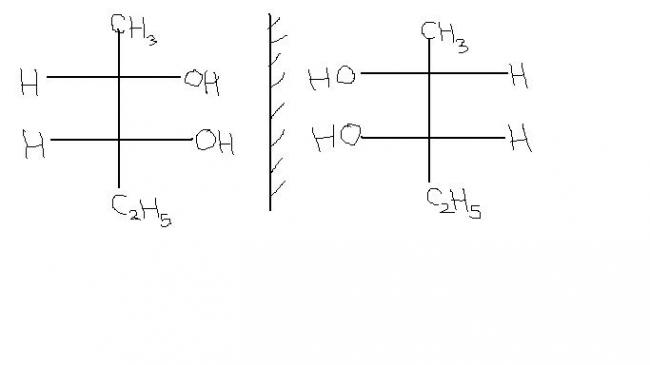 i understand that the above compounds are dissymetric. but why are they not superimposable mirror images of each other? 3.how does the umbrella effect in N-(CH3)3 make it optically inactive?
i understand that the above compounds are dissymetric. but why are they not superimposable mirror images of each other? 3.how does the umbrella effect in N-(CH3)3 make it optically inactive?