is it 4 ?

How many enantiomers and diastereomers are possible ?
(a) 2 , 2 (b) 4, 4 (c) 4,2 (d) 2,4
Enatiomers to 2 hi hai, but dbt is with diastereomers.
-
UP 0 DOWN 0 0 7

7 Answers
i think it is 4 .............not sure but as the comp has 3 chiral carbons and the formula says
2n-1 = no of disaster....
where n is no of chiral carbons........
mostle so ..
2n-1
=23-1
22
=4
so mostly its 4 ..plz check and tell whether correct?
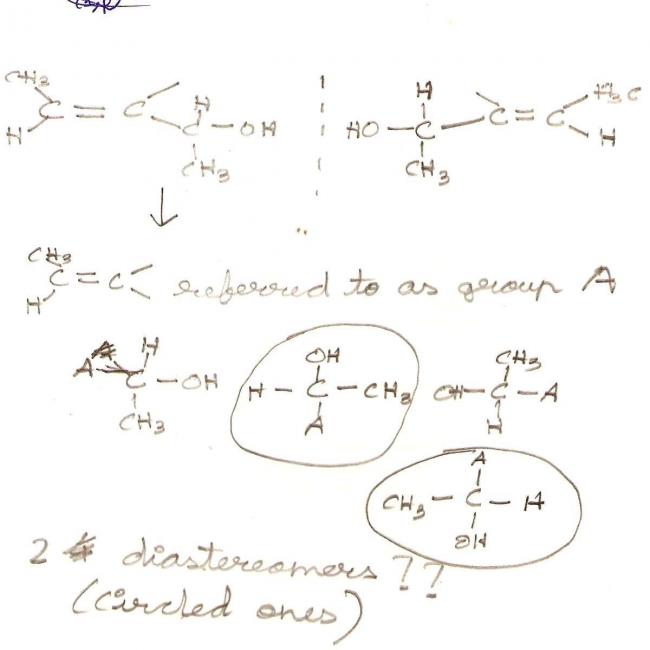
How does it have 3 chiral carbons??? I thought only sp3 hybridised carbons can be chiral..
The geometrical isomers Cis-Trans And E-Z are also considered as Diastereomers
@ Pranav....the compound has only one chiral carbon
@ Pritish ..all these isomerism part becomes more clear in 3D..in ur post atleast denote the compounds by wedge-dash notation to make it more clear...
btw....i think the compound has only two diastereomers..due to Cis and Trans
Yes govind 2 groups in same plane and other 2 away and towards observer is what I wanted to depict.