Has anyone got it?????
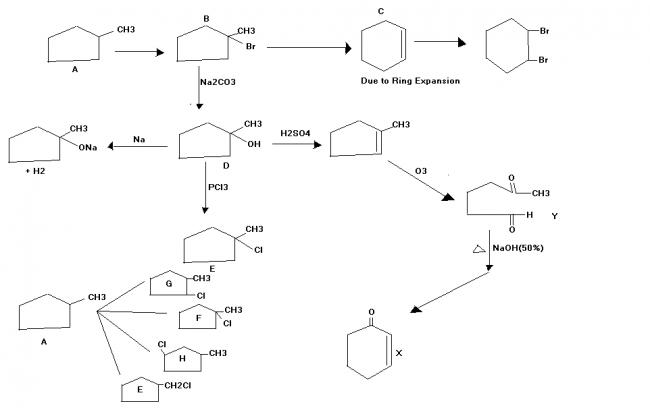
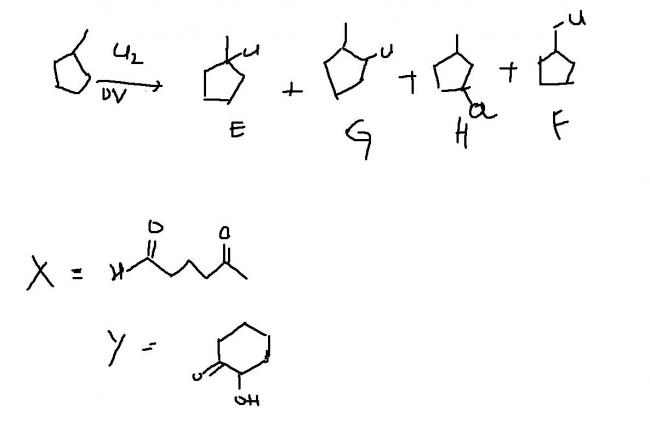
An achiral hydrocarbon A ,C6H12 was reacted with Bromine/UV light to give B as the major product.B was found to react quickly with AgNO3/ethanol and slowly with NaI in acetone.When B was heated with KO-t-Bu in t-BuOH it gave C as the major product.C reacted with Br2/CCl4 to give a colourless soln.Reaction of B with aq. Na2CO3 soln gave D,which evolves a colourless gas on heating with Na metal.Heating D with conc. Sulphuric acid gave 1-methylcyclopentene as the major product,which is a constitutional isomer of C.Reaction of D with PCl3 gave E.Reaction of A with Cl2/UV light gave a mixture of four constitutional isomers E,F,G,H in relative yields G=H>E>F.Of these four isomers only G and H were chiral.
Q1 Which compound will produce a chiral compound as on the product on treatment with EtONa/EtOH?
Q2 Which compound (C or D) will produce more heat/mole on hydrogenation with H2/Pt?
Q3 Write the structure of B.
Q4 D on reaction with conc. H2SO4 and heating followed by ozonlysis and treatment with Zn-H2O gives a product Y which on treatment with NaOH(50%) and heat gives X.Identify X and Y
PLzzzz give complete soln as soon as possible...........
-
UP 0 DOWN 0 2 24

24 Answers
sorry for replying it so late but srinath is taking good care and i beleive the solutions posted by srinath are quite very true
I'm not a master!!!!!!!!!!!!!!! . What I know is only the tip of the iceberg. there's so much to learn .
and post the answers. that you ahve let me correct myself. and I'm telling aggain . I've not answered your Q's 1-4 . I've only provided the reactions ,from them you must get the answers to the Q's 1-4 . kya kare mein to bahut aalsi hoon. ?
@ eureka . check mine da. I think mine's correct . I left some things as they were posted by gagar. ( gaurav.) especially the answering part. I've given each step there as to what happens. so , if you have any doubts on that u can ask . else . njoy finding hthe answer for the Q's posted by you . It's right in front of your eyes. just connect them.
and @ Siddharth . why does ring expansion take place from C ---> D . please give your explanation.
Answers
Q1. G and H
Q2. D (C is a 6 membered cyclic chain.It is more stable than 5 membered chain)
Q3.See Image
Q4.See image(to the right of D)
well atleast you should have tried giving your approach.
here's mine.hope it suffices.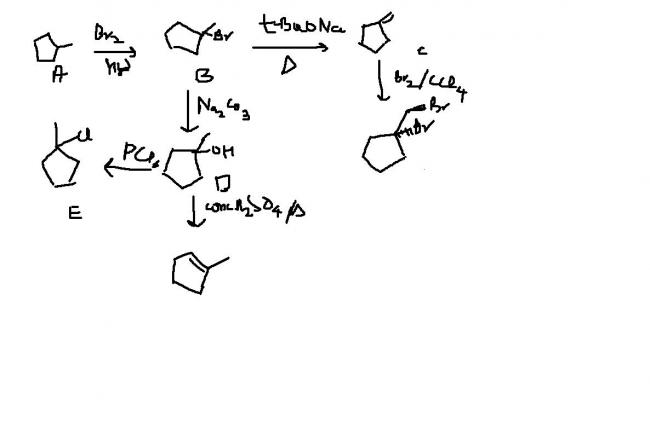
absolutely correct............................PLZZZZZ POST THE SOLUTION TO IT..........thats more important for me........
leave that srinath for the second question it is now obvious that c will be the ans
ans for the question
1→G and H
2→C
3→one bromo onemethyl cyclo pentane
4→ x is a aldol product (six membered)
hey nothing da , forget it . but my Q was how can you hydrogenate D ? that's all everything else was dumb :(
it would be better if you try it out and give your approach and why you are not getting it ,than asking for the answer right out.
tho kya... i can also mind it... [9] [3]
right of minding useless things... fundamental right [3] [4]
juzz kidding... plzz mind it... [6]