it is very complex...
can u jst tel me that on which carbon, oxygen of carbonyl will be attached and why
CH3CHCH2
10 Answers
oh these industrial processes are not needed in that detail but if u want:
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) originally referred to the oxidation of ethylene to acetaldehyde by oxygen in water in the presence of a tetrachloropalladate catalyst. The same basic reaction is currently used to produce aldehydes and ketones from a number of alkenes with the Monsanto process for producing acetic acid. This chemical reaction, a German invention, was the first organometallic and organopalladium reaction applied on an industrial scale. The Wacker process is similar to hydroformylation, which is also an industrial process and also leads to aldehyde compounds. The differences are that hydroformylation promotes chain extension, and uses a rhodium-based catalyst system. The Wacker process is an example of homogeneous catalysis. The palladium complex with ethylene is reminiscent of Zeise's salt, K[PtCl3(C2H4)] which is a heterogeneous catalyst.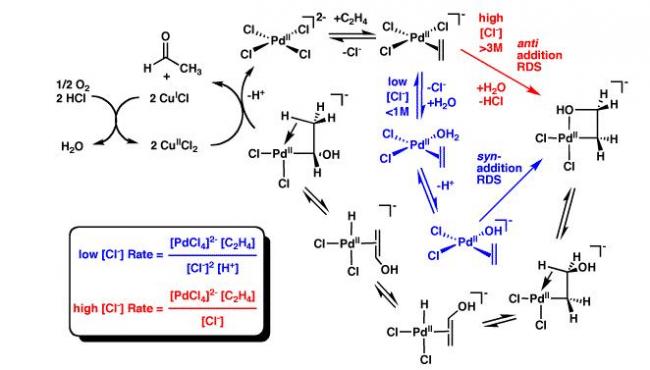
The catalytic cycle can also be described as follows:


Meachanism

here CuCl2 plays a very vital role.
It reoxidises Pd to PdCl2 , as Pd is very costly this is very necessary . also, Cu + formde is oxidised to Cu2+ by O2 thus the only oxidising agent used up is O2 and nothing else.
Guys i dont know why u guys get carried away!
Dont go into stuff that is not in IIT JEE syllabus. The new syllabus is out..
IIT JEE will never give questions which are not in syllabus. More so in subjects like chemistry. This is one subject where u should be extra careful!
The IIT JEE syllabus for this year is out. It is available here:
http://www.iitkgp.ernet.in/jee/chemistry.htm
http://www.iitkgp.ernet.in/jee/maths.htm
http://www.iitkgp.ernet.in/jee/physics.htm
http://www.iitkgp.ernet.in/jee/aptitude.htm
for Named reactions.. dont read anything beyond the list of Named Reactions!
Concepts: Hybridisation of carbon; Sigma and pi-bonds; Shapes of simple organic molecules; Structural and geometrical isomerism; Optical isomerism of compounds containing up to two asymmetric centres, (R,S and E,Z nomenclature excluded); IUPAC nomenclature of simple organic compounds (only hydrocarbons, mono-functional and bi-functional compounds); Conformations of ethane and butane (Newman projections); Resonance and hyperconjugation; Keto-enol tautomerism; Determination of empirical and molecular formulae of simple compounds (only combustion method); Hydrogen bonds: definition and their effects on physical properties of alcohols and carboxylic acids; Inductive and resonance effects on acidity and basicity of organic acids and bases; Polarity and inductive effects in alkyl halides; Reactive intermediates produced during homolytic and heterolytic bond cleavage; Formation, structure and stability of carbocations, carbanions and free radicals.
Preparation, properties and reactions of alkanes: Homologous series, physical properties of alkanes (melting points, boiling points and density); Combustion and halogenation of alkanes; Preparation of alkanes by Wurtz reaction and decarboxylation reactions.
Preparation, properties and reactions of alkenes and alkynes: Physical properties of alkenes and alkynes (boiling points, density and dipole moments); Acidity of alkynes; Acid catalysed hydration of alkenes and alkynes (excluding the stereochemistry of addition and elimination); Reactions of alkenes with KMnO4 and ozone; Reduction of alkenes and alkynes; Preparation of alkenes and alkynes by elimination reactions; Electrophilic addition reactions of alkenes with X2, HX, HOX and H2O (X=halogen); Addition reactions of alkynes; Metal acetylides.
Reactions of benzene: Structure and aromaticity; Electrophilic substitution reactions: halogenation, nitration, sulphonation, Friedel-Crafts alkylation and acylation; Effect of o-, m- and p-directing groups in monosubstituted benzenes.
Phenols: Acidity, electrophilic substitution reactions (halogenation, nitration and sulphonation); Reimer-Tieman reaction, Kolbe reaction.
Characteristic reactions of the following (including those mentioned above): Alkyl halides: rearrangement reactions of alkyl carbocation, Grignard reactions, nucleophilic substitution reactions; Alcohols: esterification, dehydration and oxidation, reaction with sodium, phosphorus halides, ZnCl2/concentrated HCl, conversion of alcohols into aldehydes and ketones; Ethers:Preparation by Williamson’s Synthesis; Aldehydes and Ketones: oxidation, reduction, oxime and hydrazone formation; aldol condensation, Perkin reaction; Cannizzaro reaction; haloform reaction and nucleophilic addition reactions (Grignard addition); Carboxylic acids: formation of esters, acid chlorides and amides, ester hydrolysis; Amines: basicity of substituted anilines and aliphatic amines, preparation from nitro compounds, reaction with nitrous acid, azo coupling reaction of diazonium salts of aromatic amines, Sandmeyer and related reactions of diazonium salts; carbylamine reaction; Haloarenes: nucleophilic aromatic substitution in haloarenes and substituted haloarenes (excluding Benzyne mechanism and Cine substitution).
I meant any name outside these!
nishant sir how will the name rxns will be useful at the examinations sir can u explain how it works in jee .and i have read only the rxns under the syllabus.
Yup sir is correct................chemistry is a subject where students get deviated the most...............
PLZ STICK TO THE SYLLABUS..............