normal addition of basic KMnO4 to alkenes brings about acids
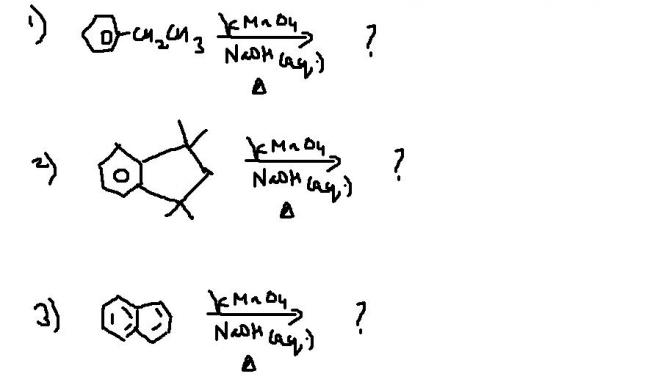
i just need the answers ...becoz i feel my answer key is wrong..
-
UP 0 DOWN 0 0 13

13 Answers
Addition of alkaline KMnO4 oxidatively cleaves the alkyl site attached to benzene and turns it into carboxylic acid, provided the group is not a tertiary one.
arre vo sab pata hai bhaiyon....apne answers post karo.....mujhe mech ya kuch aur nahin chahiye....sirf apne answers bhejo
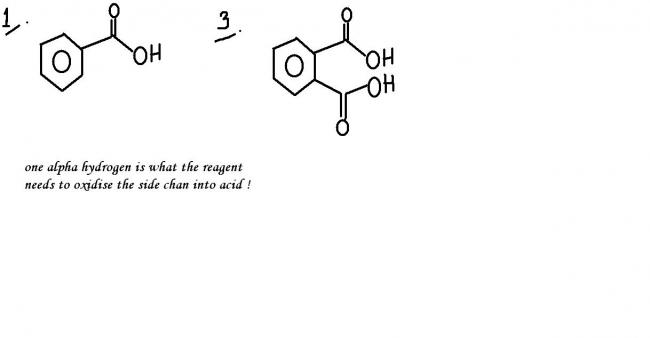
sorry...the reaction can proceed further...the base will abstract the hydrogen atoms from the acid group and prepare an anion !
Debo is spot on!
Waise will reaction 2 occur? Tertiary groups..I doubt.
i havent studied aromatic compds properly but i remem in Q2 in case of no benzylic Hydrogen , there is oxidation of benzene ring
thx debo...
i too think ans2 is no reaction...anyone else agreeing ???
debotosh, are u sure about reaction 3??? suppose instead of naphthalene, i take this: 
then which of the two rings will be oxidized???
why do u say that?? the condition for getting oxidized is that the benzylic carbon should have at least one hydrogen. that way, any of the two rings can get oxidized (if there are no other conditions involved, that is..)... sum1 plz confirm...
Shreyan the ring without Cl will get oxidised into dicarboxylic acid I think. Side chain oxidation applies to alkyl, alkenyl, etc R groups not groups with halogens attached.
k ..got the ans extreme conditions are reqd to oxidise benzene ring , wich arent metioned so reacn wont occur...tnx pritish [1]