Arihant --> 2010-2011 (white edition) :) . Please write exercise name and all like Tushar did.
Anyways, from where did you arrange old editions.. I couldn't find any second hand copy ( I didn't try to get it though)
Some simple suitable ones for practice-
1) Which of the following cannot form Grignard Reagent ?
a) CH3CH2Br b) CH2=CHBr
c) HC≡CCH2Br d) CH2=CHCH2Br
2)CH3CH2Br ---(NH3)---> CH3CH2NH2 (Given 2b true), Hence..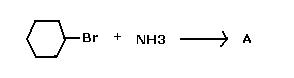
What is "A" ?

4)Most reactive towards Alco. KOH is --
a) CH2=CHBr b) CH3COCH2CH2Br
c) CH3CH2Br d) CH3CH2Cl
5)Arrange in CH3-X (X=F,Cl,I) in Decreasing order of Dipole Moments .
6) Identify Nucleophile in the following compound giving Intramolecular SN reac.n -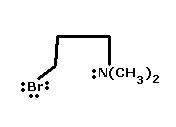
7) Select correct statement(s)-
a)Strongest force of attraction in alkyl halide is London Force.
b) London Force is a Surface Attraction.
c) Molecules with larger Surface Area have higher Boiling Point.
d)Perchloro Ethane is used as a Moth repellant.
(P.S. Some are my doubts; some are not [6])
-
UP 0 DOWN 0 1 55

55 Answers
Predict the products of the following eliminations of viscinal dibromides with KI:
a) 1,2-dribromo butane.
b) As given in your figure's a
c) as given in your figure's b
Answer : b. --> your figure's a
Well with KI the questions is damn simpler. I mean.. with KI, I is substitued in place of bromine(s) atom and then the compound is stated 'unstable' and it converts in alkene. You can check the gylcol's reaction and formation. Similar case.
Arrey , i had bought it in hope of latest, when i was in XIth :P.
n in this one there is nothing like theory, only Exercises (Chapter-wise)...
These r frm "Alkyl Halides" -
TYPE1- MCQ with only one correct Alternative)
btw ...u got 2010-1011 edition, 2010 hasn't even started yet...!!!
dunno about that.. I wondered about that too.. I bought Arihant's Differential Calculus and Organic Chemistry together and they are of 2010-2011 edition. I checked it over their site and they are selling the same (though there was no scope of fake copy like that coz every thing is authentic at Punjabi Bagh, Delhi).
But I didn't hear of any copy containing only questions from Arihant in kinda textbook . And Alas there is no Type 1 or Type 2 sort of thing in this.. there are review exercises, Cumulative exercises, Workbooks and Practice On the Spot.
I think Avik is talking abt "NEW PATTERN OBJECTIVE CHEMISTRY" by R.K Gupta and Amit
@Ankur ........... nops it's not fake [3]
@Tushar:
I know that Tushar.. It "can't be" pirated copy.. I mentioned it.. everything is original. :D .. btw.. which question bank I should prefer for Chemistry? One of my past teacher recommended me to buy 31 Previous year Questions book.. but i find it 'awe'.. I may like quesitons bank better than previous year questions..
ohk.. Is it a question bank? I mean just a question bank.. I will fancy it to buy ? Isn't there a single blend of entire chemistry: Physical, Organic, Inorganic.??
I was talking about this:
Yeah, its a Qn bank, fr complete jee chem. with diff. sort of Qn(s) - MCQ, assertion, paragraph,column... decent one i wud say...
for Q7: CCl3 CCl3 is moth repallant.. read it in preparation of polyhalides comound. (C) because, you must have read it in alkanes, when alkanes are branched, boiling point decreases, it's because it reduces the surface area and henceforth less force of interaction--> less heat required to boil.. --> less boiling point.
(B) because: London Forces is surface phenomenon (that's what i all know about London forces :D.. , I still have to cover that portion)
A) No clue.
PINK COLOURED ARIHANT
Edition 2009 - 10
Page no . 373
Practice on the spot : 9 J
Question no . 3
7. (a) is NOT correct.. in alkyl halides (except fluorides) the strongest force of attraction is dipole-dipole interaction..
London forces are dipole induced dipoles
for Q3: if it is the question as I mentioned seeing from my book (ie, KI is added).. then there shouldn't be problem in answering this question. KI is added fastly and provided that halogen is in same plane, it gives KI less opportunity to dehydrobrominate it and leaves option to substitute . Moreover, cycloalkanes generally reacts with KX to give substituted product. And when both Br are substituted with I, the compound becomes unstable (reason: weaker base on both carbon and as well as good leaving group.. tends to eliminate themselves) and forms alkene.
@Asish...no idea. It is given 2b correct. (about Q7)
@Ankur..still reading ur post.
Q1 .. terminal alkynes have acidic hydrogen which is there in (c) so as soon as Grig reagent is formed it will immediately react with the terminal hydrogen and reverse process will occur.. So (c) cant form grignard reagent
@Avik @Ashish: How (c) for Q1?
Now I'm also confused in Q3.. @Avik: Is answer given: a? cis waala? I checked in my book and it's prohibiting what I reasoned for that.. It is saying that it[cis --> a ] can't be debrominated at all.
@Ashish: But than.. if you will react acetylene with RMgX.. alkane is formed right.. (reason due to acidic H as you told) but side product will always be: HC≡C MgX ... how can this be justified by similar reasoning then? It still contains one acidic H and then accordingly it should act with itself.. throwing out Mg and being alkyl halide
see... alkyl halide is : HC ≡ CCH2 Br . Now we are reacting it with Mg. You are saying that it will react but rxn will revert back. Now I'm asking if that's the case then when we reacted CH≡CH with RMgBr we got RH and HC≡C MgBr . Now the question is if that compound's reaction is reversing.. why not it's ?
In the snippet you provided, you are focussing on the dual Grigenard synthesis to yeild BrMgC≡CMgBr.. but it still reacted..
Probably i'm wrong... bt please clarify this point.
Ohh..if its just debromination, then it will be b). Debromination can be done by treating the compound with Zn in presence of ethanoic acid, ZnBr2 and the alkene will result. Dehydrobromination is a different case, where Br has to be antiperiplanar to H.
for Q7 : even d is correct. perchloroethane: CCl3CCl3 is moth repellant. c is right for sure.. not sure about a and b.
for Q2: Qwerty is absolutely correct.
@Pritish: Please explain a bit about Q3.. It's to be debrominated. Shouldn't it be (b) then? I read it somewhere that like halogens are added to alkene with trans-addition, they are removed in similar way.
for Q1: I think, it should be (b)..
3) It is a), as the bromine atoms are anti-periplanar to the hydrogen atoms, which is a prerequisite for elimination.
6) Due to steric hindrance, -N(CH3)2 is a bad nucleophile as the lone pair becomes unavailable.
Bromine would be the nucleophile in this case. Confirm kar lo, not sure.
