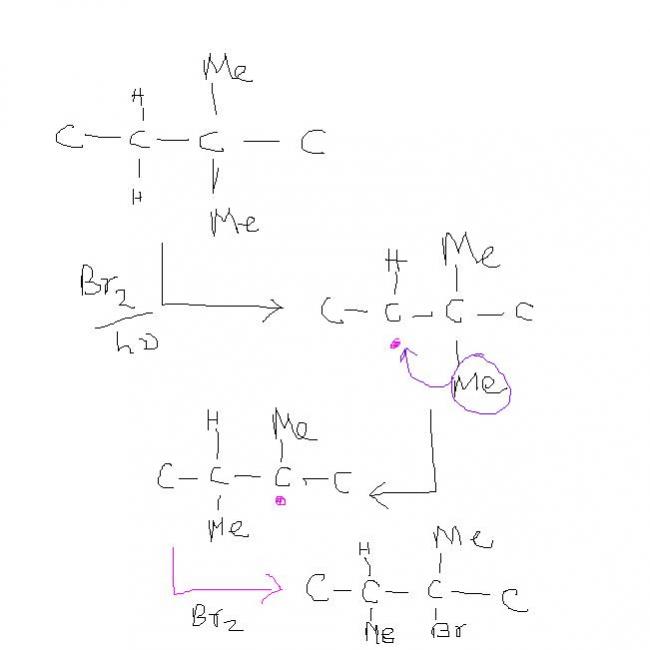
have i given the correct reason or i blabbered
The major monobromination product in the photochemical bromination of 2,2-dimethylbutane is???
-
UP 0 DOWN 0 0 35

35 Answers
why do you ppl bring in magner weermein here ?? It's not possible . the secondary hydrogen alone reacts.
no rearrangement , according to me , forgive me if I'm wrong
If you disagree , then give some proof. I'd like to know of some source which says that this thing is possible . thanks in advance.
@sky
free radicals are very very reactive , they react readily with another free radical to get a product and so they didnt have enuf time to get rearranged.
YUP! I WAS WRONG IN MY PREVIOUS POST...
THANX TO ALL OF YOU FOR CORRECTING ME!!
I READ IT JUS NOW IN MORRISON ...
REARRANGEMENT NOT POSSIBLE IN FREE-RADICALS ...
TX AGAIN :)
i dun think u blabbered...
reason not given .. [not given in the part i read]
only evidence given ....
kk srinath :)
@sankara ... no i havent completed ... but read almost ...
missed many things as well ... like this one... its an imp thing i overlooked :( ... tx u guys :)
hi dude,
i guess one of the primary H must get replaced as photochemical bromiantion occurs by free radical if i'm not wrong....
am i on the rite track then i'll think wether its the one on 1st carb or the 4th.....
else [2]
yup mathematics u r correct..........can u give the mechanism........plzzzz[1]
thanks dude.
nothing great, bromine is highly regioselective. use that fact and u will get it.
believe me , it's not possible . even if you think out of the box. I'm quite sure on this. onyl secondary hydrogen will be substituted.
actually i am confused about reactivity of Bromine.......I know its higly selective....but not in detail..........can anyone of u ,plz explain any arbitrary reaction in detail????plzzzzzz[1]
yo mathie rite [4] ......
i forgot dat again???
ur name shud be CHEMISTRIAN!!!! LOL....
the selectivity factor for BROMINATION
P: S: T
1: 82:1600
WHERE P IS PRIMARY HYDROGEN
S IS SECONDARY HYDROGEN
T IS TERITARY HYDROGEN