first Cl-Cl --> Cl.
.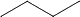 + Cl. -->
+ Cl. --> 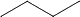 + HCl
+ HCl
So the free radical formed is now planar
.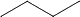 + Cl-Cl -->
+ Cl-Cl --> + Cl.
+ Cl.
So, free radical can attack the Cl-Cl such that Cl adds above the plane or below the plane.. Resulting in a racemised product
How is a racemic mixture formed when n-butane is mono chlorinated?
first Cl-Cl --> Cl.
.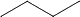 + Cl. -->
+ Cl. --> 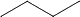 + HCl
+ HCl
So the free radical formed is now planar
.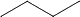 + Cl-Cl -->
+ Cl-Cl --> + Cl.
+ Cl.
So, free radical can attack the Cl-Cl such that Cl adds above the plane or below the plane.. Resulting in a racemised product
This should be the case in every free radical chlorination then,shouldn't it?
unless the substrate is too highly strained for the free radical to be planar. remember highly strained free radicals tend to have sp3 hybridisation as in carbanions. Further there is neighboring group effects also.. In general for simple compounds monochlorination usually leads to a racemised product
free radical formed is sp2 hybridised and hence is planar.
the attack of cl free radical can take place fron either side of plane .
hence racemoc mix is formed.