yes, i agree too.
(The hyperconjugative resonance being almost the same, we shud go with +I dominance i think.)
11 Answers
Bridgehead nitrogen in second, it can't be planar. So that expels it's lone pair more outward and helps in its attack.
In the tertiary amine(first) steric crowding of the lone pair decreases basicity.
but in tert,case also +i effect of et repells the cloud on N which results increase in basicity,,,
so i think it will be more basic
bhai wo - I nahin + I hota hai.................. et ka +I matlab donating effect hota hai
if it wuld hav attracting capacity then it wuld hav been - I
but et has donating effect isliye +I effect kahte hain use
Reopening the debate here.....
There is a little problem with my earlier assertion. None of you noticed that if the nitrogen on the bridgehead is attacked, it becomes planar. In that case wouldn't basicity diminish? (Bridgeheads can't be planar, so chances of attack are very small)
I think both are quite hindered bases.
manmay its a silly mistake but understand the concept..
i hope my solution is correct..have to consult manish bhaiya
Souvik +I effects will not contribute to basicity here...those bulky groups shield the lone pair from being attacked by acids.
i dont agree with pritish but i have seen solution in a book which says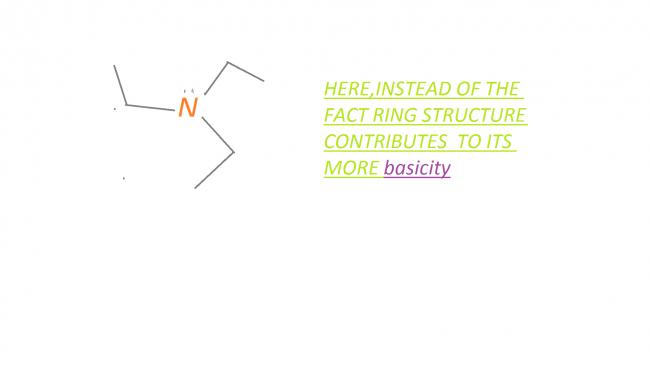
Solutions to INCHO 2009 give answer as the bridgehead nitrogen as more basic. (and i feel pritish's reasoning is correct)
Chalo phir my first reason is correct...I guess that's the final word.
