this is the first concept in organic chemistry i really loved!! [1]
prerequisites::
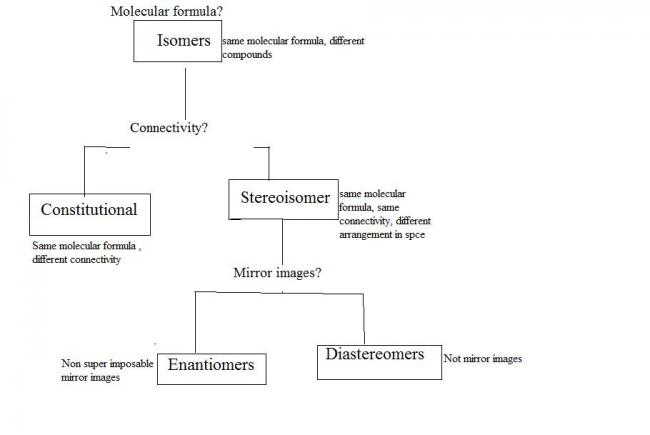
racemic mixture: a 50-50% mixture of two enantiomers...as a result though both enantiomers are individually optically active, the racemic modification is optically inactive!!
enantiomers are equivalent in all physical properties except the angle through which they rotate plane polarised light!! if one is dextrorotary the other is laevorotary!! thus their mixtures cannot be separated by any physical means..
diastereomers are stereo isomers but not mirror images of each other...as a result they have different physical properties and their mixtures can be physically separated!!
resolution: separation of racemic modification to enantiomers...[1]
majority types of resolution depend upon the reaction of organic bases with organic acids to yeild organic salts!!
let for examle we need to resolve the racemic modification of an acid (±)-HA
using an optically active alkaly like substance known as alkaloids extracted from plants... let the BASE = (-)-B
on reaction with the acid, the base forms two salts..
SALT-1:[(-)-BH+ + (+)-A-]
SALT-2:[(-)-BH+ + (-)-A-]
SALT-1 and SALT-2 are:-
a) non superimposable because the acid parts are not superimposable. thus these are stereo isomers.
b) not mirror images as the base parts are not mirror images. thus they are not enantiomers.
they are stereoisomers but nit enantiomers. so they are diastereomers...diastereomers differ in physical properties and thus are separated..
now the separated salts are treated with acid (H+) to give the respective enantiomers of the racemic acid taken and we get the alkaloid back as a salt...[1]
bases used: i) (-)-brucine ii) (-)-quinine iii) (-)-strychnine iv) (+)-chinchonone
acids used: like (-)-malic acid..
to resolve alcohols is tricky since they are neither appreciably acidic nor basic..so, we attach an acid HANDLE to the alcohol to form salt by reacting with bases, and when no more required the handles are removed...
BASIC PRINCIPLE:: A racemic modification is converted by an optically active reagent into a mixture of diastereomers which can then be separated..[1][1]
i hope this is complete....additions and editions are welcome...[1][1]