SIDDHARTH i know dat it was wrong becos it was rong as per NCERT but i dont know y it is rong
can u explain
Where did u find this reaction bro?? Nitromethane loses proton and when treated with alkali forms an acinitro compound which on treatment with sulphuric acid gives aldehyde. 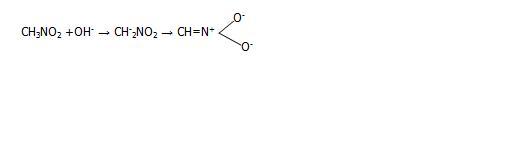
SIDDHARTH i know dat it was wrong becos it was rong as per NCERT but i dont know y it is rong
can u explain
presence of nitro on methyl group make the hydrogen on methane acidic. so they readily react with alkali to lose a proton..[1]
yup u r rite this is the expn given by NCERT also
but the resonance structure shows dat nitrogen atom has gained a +ive charge and so the OH- ion can attack directly at the nitrogen atom wont The nitrogen atom behaves like a carbonyl carbon
do consider chemical bondnig aspect da. you seem 2 b forgetting it everywhere.
greater the e.n difference stronger the bond. so CN bond id much stronger than NO bond.
and anyways CH3- is a bad leaving group.