i wont be in the same plane..
20 Answers
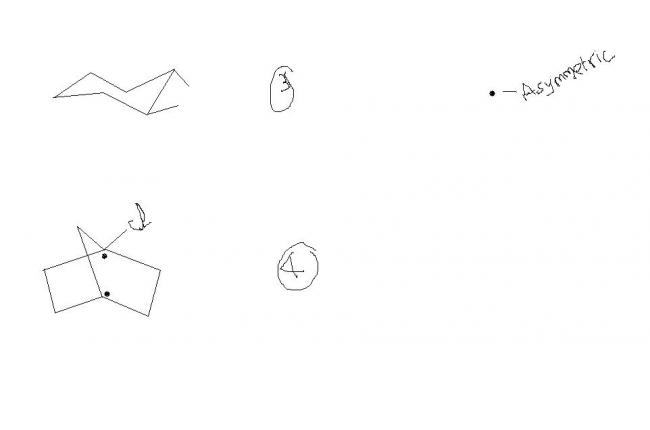
4.......d assymmetric carbon is sp3 hybridised......so Cl and d bridged C are not in d same plane.......so if u consider a plane through them u wont get any plane of symmetry.............
and first THEY ARE IDENTICAL..............just imagine a little....tilt them twist them......u twist urself.........turn d comp screen....
at last ull find them they r same
no aditya... there may b another plane of symmetry(wat i meant here is through cl atom)..even if one plane is present den it is inactive.....
@aditya.... see the other brdge havin 1 carbon....it also cuts chlorine into 2 halves
How der's a plane of symm in 4th...r both sides of the bridging C in the same plane?
fr q 1 rotate d bon d n u get d mistke cis trans r formed by bond breaking n bond formation
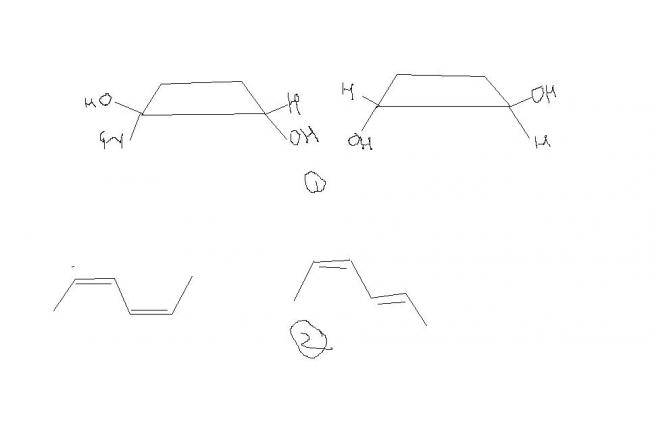
1 -- How r they identical , i thought them to be cis-trans isomers(it is para Q , n it was given even cyclic cpds exhibit geo iso.. so dont search 4 double bond)
2---Give the EZ notation for these 2
3---How dis cpd is meso cpd??
4---In dis there is a plane of symmetry passin through bridging c atom and chlorine ( am i rite??) but it was given as optically active.....