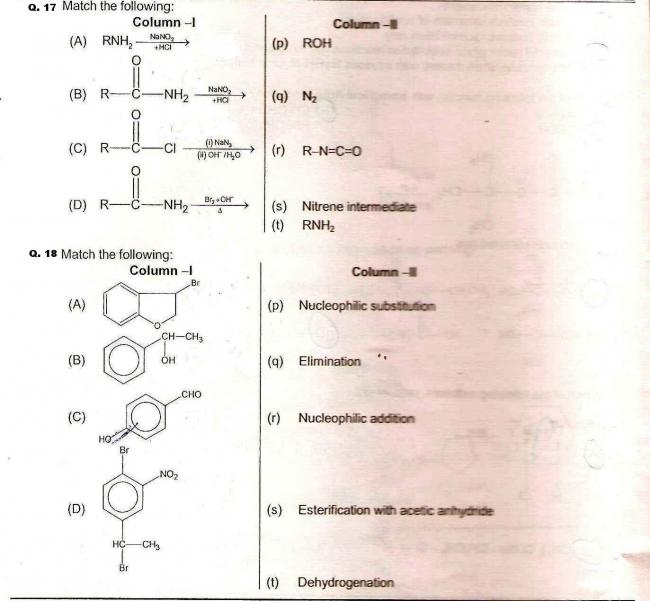
Q17)
A → p,q
B → q
C → q,r,s,t
D → r,s,t
Welcome to the Great Organic Chemistry Challenge kiddos. Let's see what you got. Give it your best shot. You should know that I don't have the answers to any of these questions. Don't bank on me. Wake out of your slumber and stop wasting time on optical isomerism. There are some places in questions where I will have to help you, but you must post queries on those portions first! Atleast I would know that you have tried yourself upto that point.
For all those indulging in optical isomerism, this speech(inspired by Sylvester Stallone from Spy Kids 3 : Game Over) :
"I'm coming back
To settle the score...
For imprisoning me
For uuusing me
So now...I'm going to take this to the next.level!!!"
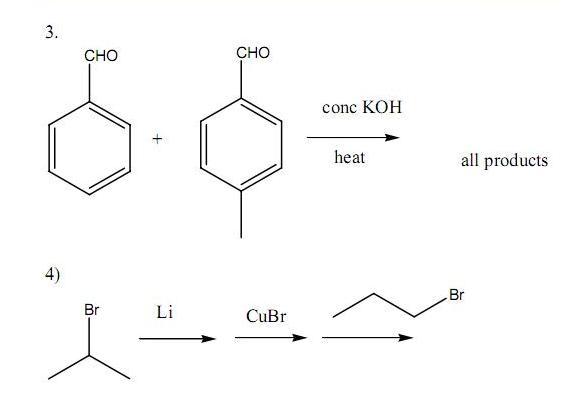
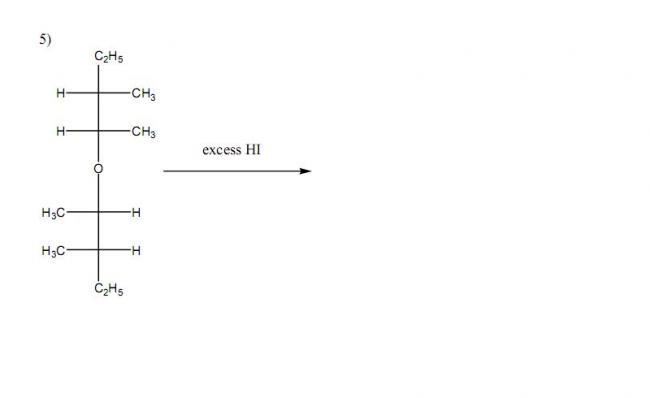
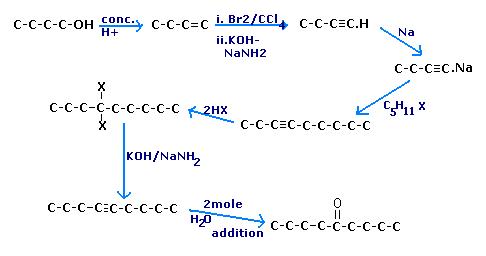
Q6. Using 1-propanol as the precursor compound, synthesise the following compounds in good yield(try using the shortest possible number of steps) -:
a) Hexan-3-one
b) 4,5-diethylocta-4-ene (high yield)
c) 4-ethylheptan-4-ol
d) hexan-1,4-diol
e) Methane
f) Crotonaldehyde
Q7. An unknown organic compound X, with molecular formula C6H10O3 is optically active(your love of optical activity has brought us to this conclusion, subho).
When X is reacted with acidic dichromate, it turns green.
When X is reacted with per-iodic acid, compound A with molecular formula C4H6O3 and B, C2H4O2 are formed.
B is found to be a carboxylic acid. A responds positively to Tollen's test and negatively to the iodoform test. Find X, A and B and identify the reactions and products.
Q8. 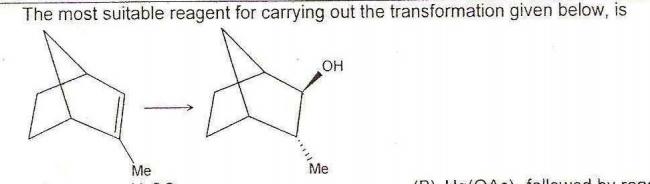
A) Aqueous sulfuric acid
B) Mercuric acetate followed by reaction with Sodium borohydride
C) Diborane followed by hydrogen peroxide/NaOH
D) mCPBA followed by H2SO4
Q9. The major product obtained on reaction of CH3COCH2COOC2H5 with one equivalent NaH and one equivalent methyl iodide is?
Q10. The reaction of phenylacetylene with one equivalent of methyl magnesium bromide followed by reaction with benzaldehyde and hydrolysis yields?
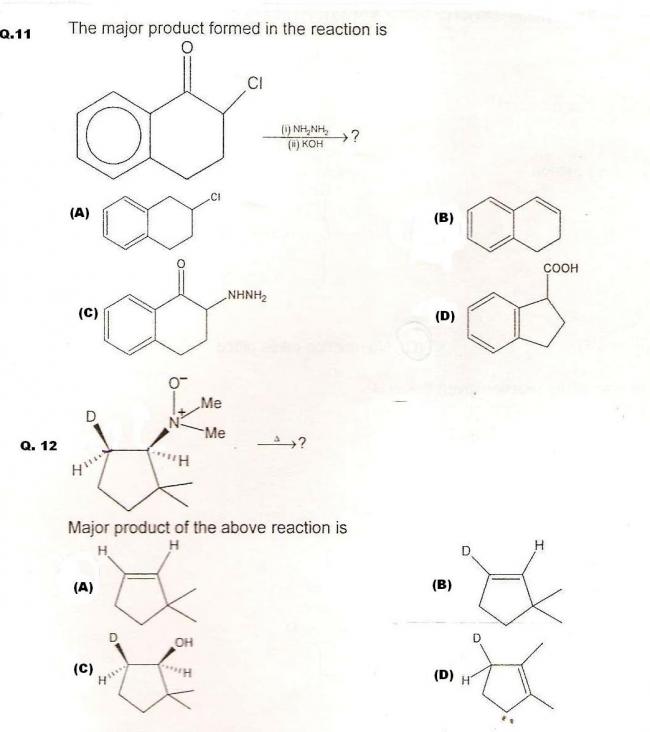
Q13. Which of the following organic compounds will not give foul odour of isocyanide on heating with chloroform and potassium hydroxide? (may be MCQ)
(A) para-methylaniline
(B) H2N - CH2 - COOH
(C) meta-aminobenzoic acid
(D) para-aminosulfonic acid
Q14. When α,Β-unsaturated cyclohexanone is reacted with (CH3)2CuLi and hydrolysed, the product obtained is?
Q15. Which compound undergoes decarboxylation on warming?
(A) Me-CO-COOH
(B) Me-CO-O-Et
(C) Me-CO-CH2COOH
(D) HO-CH2-COOH
Q16. Which of the following reacts with ammoniacal AgNO3 solution? (MCQ)
(A) 1-butyne
(B) MeCHO
(C) HCOOH
(D) PhCOPh
Q19. Explain why gyloxal gives the Tollen's test but not the Fehling's or Benedict's test.
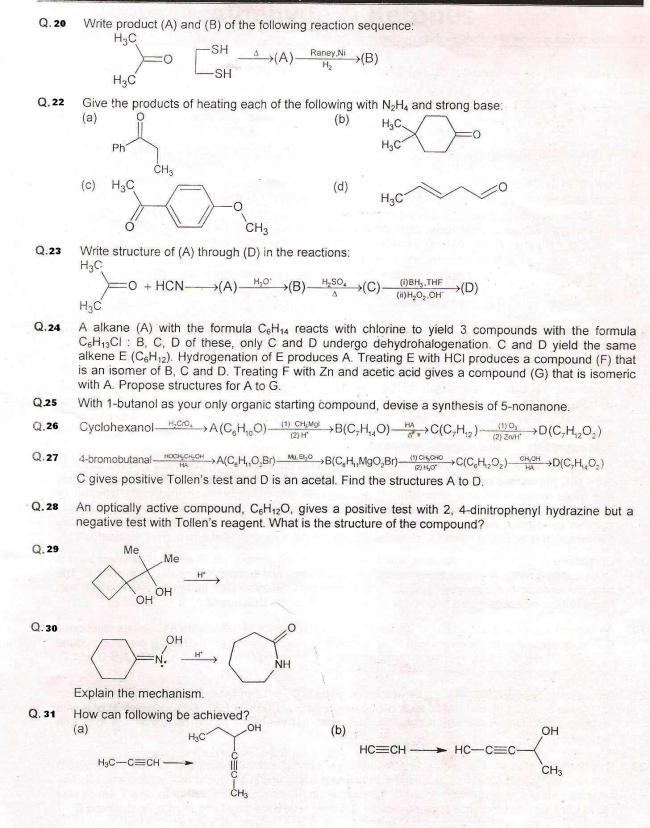
Q37. An organic compound A(C8H16) on ozonolysis yields aldehydes (B) and (C). While (C) performs Cannizzaro reaction, (B) can undergo aldol condensation. B on treatment with excess HCHO in presence of base gives (D) with formula (CnH20O√n). (B) on treatment with red P+HI yields an alkane which contains 1°, 2°, and 4° carbon atoms. Find the structures.
Q38. An organic aliphatic compound (A) when treated with anhydrous ZnCl2 + conc HCl produces a turbidity in 7.5 minutes. (A) decolourises Br2 in CCl4. (A) when treated with ThO2 gives (B). (B) on treatment with 50% H2O2/HIO4 produced three aldehydes - (C), (D), and (E). (C) on treatment with 50% NaOH gave an alcohol of least molecular weight along with sodium salt of an acid (F). (F) is peculiarly capable of reducing Tollen's reagent. (D) on treatment with MeMgBr followed by hydrolysis gives a secondary alcohol of least molecular weight. The formula of (E) is C6H10O2.
(A) on treatment with H2O2/HCOOH produced a compound of molecular formula C9H20O3. (B) on hydrogenation using [RhCl(PPh)3] produces (G). (G) on ozonolysis produces (D) alongside (H). (H) on Clemmensen's reduction produces diethyl dimethyl methane. Identify A to H.
It would be unfair if I did not give some hints here.
1. ThO2 dehydrates alcohols almost exclusively by the Hoffmann route.
2. Hydrogenation by RhCl(PPh)3 or Wilkinson's catalyst exclusively reduces a terminal double bond without affecting internal unsaturations
More to come.
The one who posts the best answers will get a toffee.
Solved Questions/Credit/Hall of Fame :
Q1. Solved by ayush_gupta
Q4. Solved by AnkurG
Q3. Solved by AnkurG
Q8. Solved by AnkurG
Q12. Solved by Manmay (http://targetiit.com/profile/manmay-kumar-mohanty-4967.html)
Q5. Solved by Avik (http://targetiit.com/profile/avik-4025.html)
Q13. Solved by Manmay (" ")
Q15. Solved by Avik (" ")
Q7. Solved by Avik(" ")
Q16. Solved by AnkurG
Q2. Solved by AnkurG
Q30. Solved by AnkurG and perfected by Avik
Q31. Solved by AnkurG
Q29. Solved by AnkurG
Q28. Solved by Avik
Q20. Solved by AnkurG
Q23. Solved by AnkurG
Q24. Solved by Avik
Q17. Solved by Manmay
Q22. Solved by Manmay
Q11. Solved by Manmay(Perfect!!)
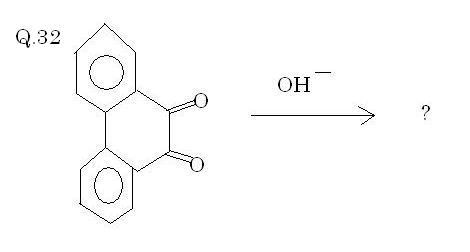
Q32. Solved by AnkurG(Bloody brilliant I say)
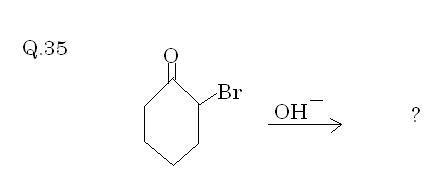
Q35. Solved by AnkurG(fastest answer)
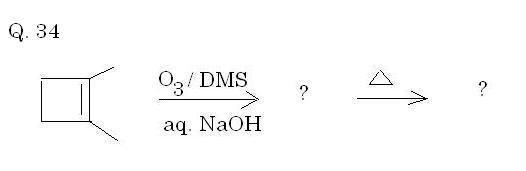
Q34. Solved by AnkurG(this is getting tedious indeed!)
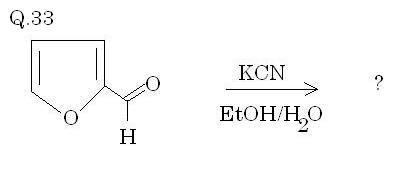
Q33. Solved by Manmay
Q9. Solved by Avik
Q26. Solved by Avik
Q37. Solved by AnkurG
-
UP 0 DOWN 0 2 87

87 Answers
Q22)
(a) Ph-CH2 - CH2-CH3 [ wolff kishner ]
(c) CH3-CH2-Ph-OCH3 [ wolff kishner since -0CH3 isn't a base sensitive group so remains as it is ]
(b) 1,1 - Dimethylcyclohexane
(d) CH3-CH = CH - CH2-CH3
@AviK: I didn't notice earlier,.. but what was the mistake in your Beckmann's rearrangement?
Arrey no Ankur, am not into toffees at all.... more into cakes & ice-creams rather ;P
So, rather than the toffee stack passing on further frm me to smone else,,, its better pritish keeps it safe.
We both will strive on Modesty & continue the gud work anyways. ;D
@Pritish, Sry fr the blunder in Beckmann (dammit yaar...how cud i do smthing like tht..?! Huh! )
Edited Now.
don't worry Avik, I'll undoubtedly forward entire stack of toffee to you.. No need to feel disheartened. I have given up my passion of toffees since 5 yrs back. :P
PS: Aah!! How modest I'm.. ROFLMAO!
Avik...the mechanism for Beckmann is wrong :P I just noticed!
[4]...Yeah, i'm going fr a nap now.
Waise Pritish, send Ankur the toffee already, kyunki it will take 3-4days to reach at least... [3]
Considered.
Now let the other damn kids try this already! Why isn't anyone else trying??
Hide your correct answers btw...let the images be.
24)
A- 2,2-DiMethyl Butane
B- 1-Chloro- 2,2-DiMethyl Butane
C- 2,2-Dimethyl-3-CHloro Butane
D- 1-Chloro-3,3-dimethyl butane
E- 2,2-Dimethyl but-3-ene
F- 2-Chloro-2,3-dimethyl butane
G- 2,3-Dimethyl Butane
Now u sit n figure out the structures :D
Q23: firstly CH3-C(CH3)(CN)OH , then by hydrolysis COOH in place of CN, then by H2SO4, dehydration to give CH2=C(CH3)(COOH). then HBO reaction to give two products :
CH2(OH)-CH(CH3)(COOH)(40% yield) and CH3-C(OH)(CH3)(COOH) (60% yeild).. This question was in my module and these values were mentioned.
Q20: it's simple mozingo method for preparation of alkane.
product: propane.
28) Is this-
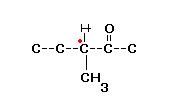
(account fr the rest of the Hydrogens plz.)
Gives 2,4DNP so Carbonyl +nt, Dosen't give tollen's therefore Ketonic carbonyl Gr.
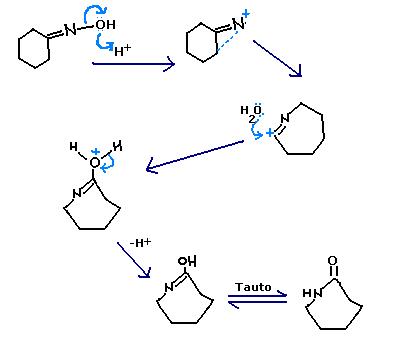
Q30: Simple! It's Beckmann rearrangement of oxime. making structure is difficult in paint.
PS: Making structure is gruesome task!
Q31 --> a) reactant + NaNH2 + propanal + H2O --> product
b) reactant + formaldehyde + NaNH2 + H2O --> Product.
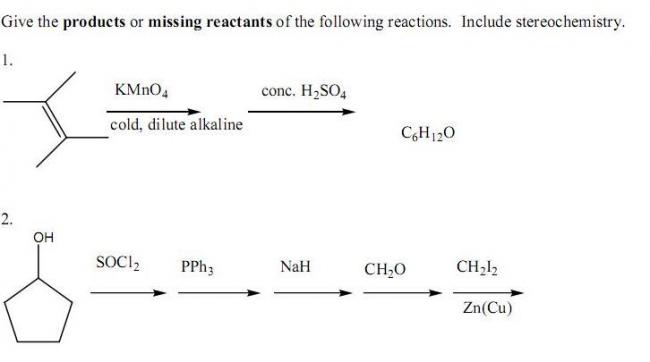
For Q2:
Step 1: replace OH by Cl
Step 2: PPh3+-cyclochloropentane
Step 3: PPh3-cyclopentane
step 4: Wittig reaction to give CH2=cyclopentane
step 5: addition of CH2 at dpuble bond
Final: 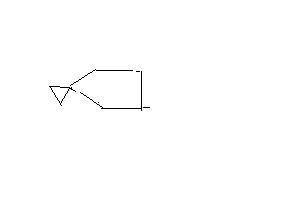
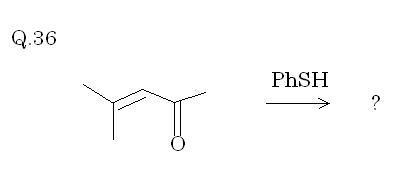
Q 36 . It ' s the good old " Michael Addition " , since thiophenol reacts with α , β unsaturated carbonyl compounds via " Michael Addition " . I 'll show the product if required right now too sleepy : )
Q2 . 
First , normal halogenation of the ring would take place , followed by the formation of a phophorus salt . When treated with a base , it easily forms an " ylide " , i.e , the starting compound of the next reaction , the " Wittig " reaction . Finally , it's just normal " Simmon's - Smith " reaction .
Q37:
Hereby solving this question as I did myself on the given basis of hints:
Hint 1: A gives B and C on ozonolysis. C gives Cannizaro, B gives Aldol.
C: HCHO
A: C8H16 ---> C7H14=CH2
B: Compound is aldehyde (as mentioned) and moreover, it ought to have atleast one alpha H. I started it with two alpha Hs.
Hint 2: IN excess of HCHO and presence of base.
This was the complex step and took lot of time to get solved (though being easy).
Let B be : C5H11-CH2-CHO.
on HCHO and base.. first step will be preferentially crossed aldol.
Stage 1: (CH2OH) CH(CHO)(C5H11)
Stage 2: (CH2OH) (CH2OH) C(CHO)(C5H11) // Similar crossed aldol.
Stage 3: Here comes the twist in the story. The product obtained after stage 2 doesn't have any alpha H present.. but HCHO is still in excess. So what will happen is crossed cannizaro in product of Stage 2 and HCHO.
Stage 4: (CH2OH)3C(C5H11)
Counting H : 20
Counting C: 9
Counting O : 3
Given Condition: CnH20O√n
---> Condition Satisfied.
Now..
Hint 3: B on treatment with Red P / HI gives 1 degree, 2 degree and 4 degree C in alkane formed.
As 4 degree is mentioned, it means, that a carbon must be surrounded with Hydrocarbons from all four sides.. this helps in solving out C5H11 in deduced B C5H11-CH2-CHO.
Structures possible:
C-C(C,C)-C-C-CHO or C-C-C(C,C)-C-CHO
Hence solved.
Another way for Q25 which escaped us all....
C-C-C-C-OH + P.C.C ----> C-C-C-CHO
C-C-C-CHO + KMnO4 ---> C-C-C-COOH
C-C-C-COOH + Ag+/CH2N2 ---> C-C-C-C-COOH
C-C-C-C-COOH + PCl5 ---> C-C-C-C-COCl + Pd/BaSO4 ---> C-C-C-C-CHO
C-C-C-C-CHO + (C-C-C-C)MgBr + H3O+ ----> C-C-C-C-C(OH)-C-C-C-C
C-C-C-C-C(OH)-C-C-C-C + Al(OR)3 ----> C-C-C-C(=O)-C-C-C-C-C
The last reaction is the opposite of Meerwin-Pondorf-Verley reduction and is known as Oppenauer oxidation. It is effective for all alcohols(primary sec tert) as it involves use of aluminium catalysed shift.
Grignard reactions :D
I would say there is an easier method to this...could not help myself from posting :P
C-C-C-C-OH + KMnO4 ---> C-C-C-COOH
C-C-C-COOH + CaO/NaOH ---> C-C-C
C-C-C + Cl2/hv ----> C-C-C-Cl
C-C-C-Cl + C≡C-C-C-C-C + NaNH2 ----> C-C-C-C≡C-C-C-C-C
C-C-C-C≡C-C-C-C-C + HgSO4/H2SO4 ---> C-C-C-C=C(OH)-C-C-C-C
C-C-C-C=C(OH)-C-C-C-C -------> C-C-C-C-C(=O)-C-C-C-C
Formation of a 9 carbon skeleton in 6 steps! Ha!
(5 if you discount the automatic tautomerization :P)
And avik...in Q18, do consider dehydrogenation :P
Okay....with which option the problem is ? :P
Here's my trial fr Q25)-
( Though a shorter method may exist; but still smthing's better than nothing :D )