can u xplain asish how (c) u went for ??
I dont have answers for even a single question...so I request all for a healthy and fruitful discussion [1]
-
UP 0 DOWN 0 1 19

19 Answers
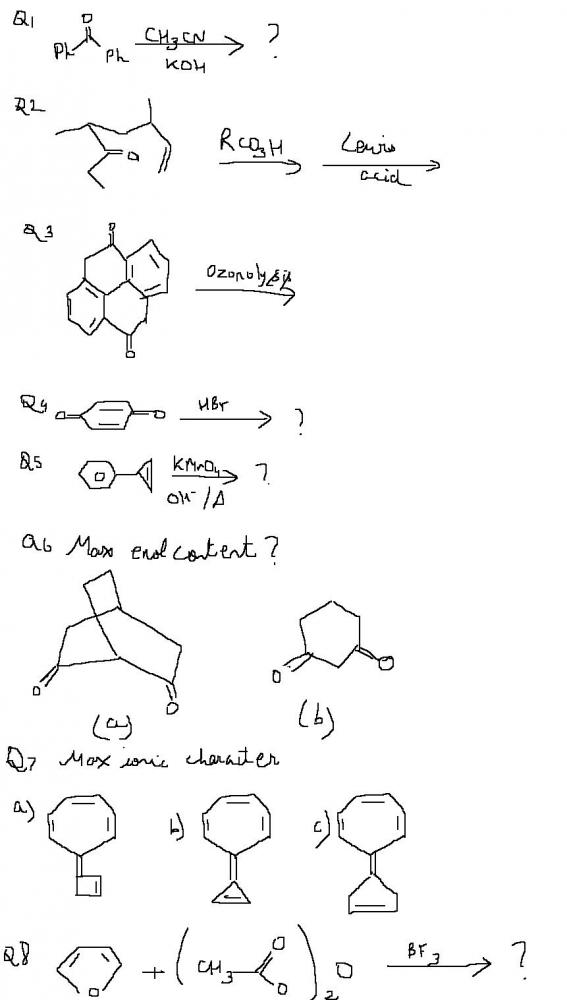
Q5 no reaction? Benzylic carbon is tertiary. No oxidation.
Should be some reaction though, heat bhi hai aur strongly basic medium hai. Then I wonder what..
Q7. I would go for C (@iitimcomin: aromaticity can never be induced in the five memberd ring whereas in the rest one ring becomes antiaromatic)
Q6. B (Conjugated product can be formed In A conjugation not there)
4) ...... just a guess...
benzoquinone may be :P a good oxy agent.....
so it gets reduced to phenol and oxy. HBr to Br2
RCO3H/Lewis Acid is reagent for BAEYER VILLIGER OXIDATION of ketones to esters.
pritish in 2 only if R is some bulky stuff epoxide will remain na .... and wat fxn of lewis acid??
Q6 B .....guessin .... because of the no sp2 carbon on bridgehead funda
nahin yaar...reaction ho rahi hai...aur ans kuch diff bhi hai..if u want ..i cna post the ans..and u cna help me with mech
Q7 C) ...[- on 5 mem ring and + on seven mem wil make both aromatic]
@pritish....can u help with a fig plz for both the ques u r talking abt ??
and ya for ur "CID" type dbt....[3]dont mind plzz
ques posted at Posted 6:34pm 22-03-10
and i gave u teh ans at 7:18pm 22-03-10
when i posted i didnt have single ans....but during that time i arranged the answers......I hope the curious case of missing ans is solved now [6] :P
EUREKA! (not calling you, general expression lol)
I was thinking of the same thing!! It's not strange at all, it makes perfect sense.
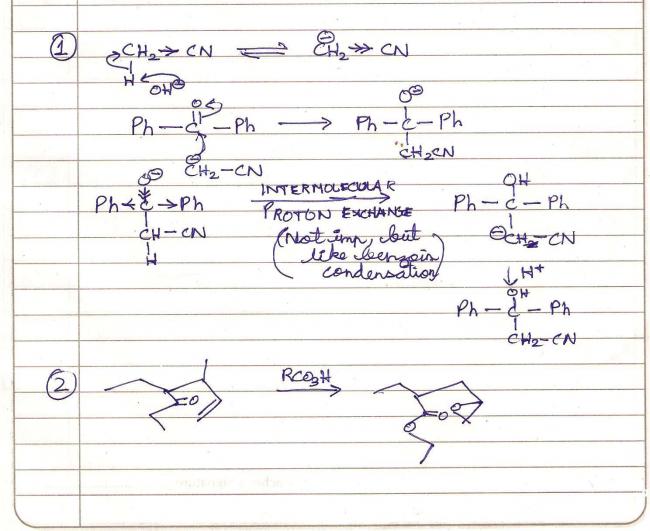
Base KOH will abstract proton from methyl cyanide as CN has a powerful enough inductive effect to make methyl protons acidic. This becomes a carbanion and adds as nucleophile to Ph-(C=O)-Ph, hydrolysis of which gives your answer.
In Q2, RCO3H has a dual function. It oxidises ketone to ester(Baeyer Villiger oxidation) and transforms alkene into cyclic ether. Lewis acid is part of reagent (RCO3H + Lewis acid facilitate Baeyer villiger oxidation of ketones).
But eure...
"I dont have answers for even a single question...so I request all for a healthy and fruitful discussion :)"
And then
"ans1 is strange as always.."
Am I missing something here? :P
Q1 is mein reaction nahi hoga, tushar is right. Benzoin condensation is possible only if that carbon atom carries a proton...how will we generate a carbanion for the rest of the reaction?
Edit : Sorry, benzoin condensation is by KCN..lol.
Ans 8 ) Furan..Friedal Crafts...it will occur at the position just next to O.. -COCH3 grp will be attached