My first question is how bond angle is related to pKa values of a compound....Heres d question
Q1. Selected bond angles for five hydrocarbons are shown below. Arrange these hydrocarbons according to their pKa values, from lowest to the highest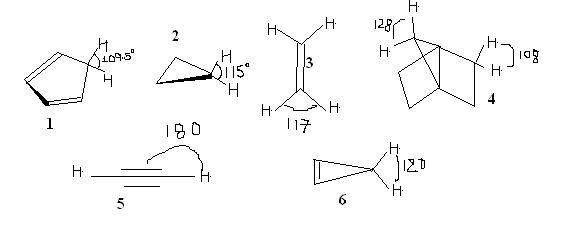
-
UP 0 DOWN 0 0 3

3 Answers
Maybe after losing H+ , the angle between the lone pair of electron and the bond pair matters...te greater tha angle..more stable will be the conjugate base..so acid will be strong but i think we will also have to take into account resonance after removing the H+
 Anurag Ghosh Resonance toh sab mein nhin hoga......so first of all should we eliminate d compounds in which resonance is possible as they will be d most acidic?.......and still didn't cleared d concept about d bond angle and pKa values.....Upvote·0· Reply ·2013-12-11 06:28:30
Anurag Ghosh Resonance toh sab mein nhin hoga......so first of all should we eliminate d compounds in which resonance is possible as they will be d most acidic?.......and still didn't cleared d concept about d bond angle and pKa values.....Upvote·0· Reply ·2013-12-11 06:28:30 Akshay Ginodia Anurag ..sir sahi bol rhe h..reso and hybri se compare krte h..using bond angles for acidic characters is irrelevant
Akshay Ginodia Anurag ..sir sahi bol rhe h..reso and hybri se compare krte h..using bond angles for acidic characters is irrelevant

Bond angles are not the main criteria for comparing acidic character. Use resonance and hybridization to compare
Shouldn't this be the order-
In terms of Ka values- 1>6>5>3>4>2
So,pKa must be- 2>4>3>5>6>1?????
Was confused between 2 and 4.....according to my logic 2 is more strained than 4,so bringing positive charge on 2 is of no use and makes it more unstable.....So,acidity of 4>2???