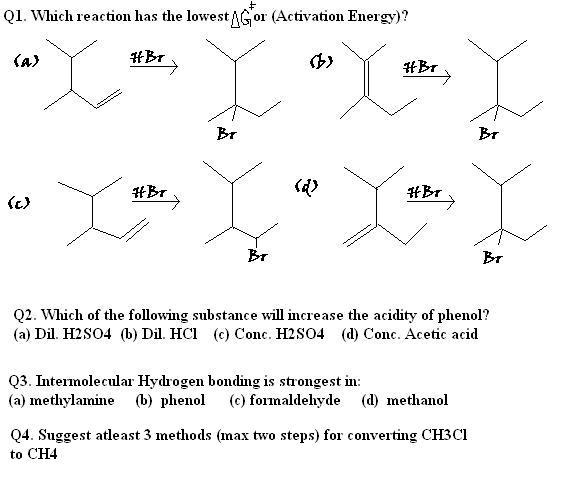
5 Answers
For Question 1
They are forming the same carbocation, So greater the energy of the reactant lower is the Activation Energy.
In B reactant is more stable than that of D. So difference of energy between the carbocation and reactant will be greater in B
Q1. (d)
Q2.Maybe (c)
Q3.I think (b)
Q4.(a)CH3Cl + Ni(300°C)→CH4
(b)CH3Cl + Zn-Cu couple/C2H5OH→ CH4
(c)RX + Red P/HI→ CH4
 Akshay Ginodia Sry the answer to Q1 should be (b)
Upvote·0· Reply ·2013-12-11 04:38:22
Akshay Ginodia Sry the answer to Q1 should be (b)
Upvote·0· Reply ·2013-12-11 04:38:22 Anurag Ghosh 1.answer given is d
2.c...u r rite
3.b...again u r rite..but y not methanol,is it depended on more electron pushing group???
Anurag Ghosh 1.answer given is d
2.c...u r rite
3.b...again u r rite..but y not methanol,is it depended on more electron pushing group??? Akshay Ginodia in methanol the polarity of O-H bond is less due the +I effect of -CH3
Akshay Ginodia in methanol the polarity of O-H bond is less due the +I effect of -CH3
aur 1. ka i had doubt on (b) and (d)........in (d),there is a possibility of forming a 1 degree carbocation,which is les stable dan 3 degree,which increases d activation energy.......
But b mein both d possibilities are 3 degree carbocation.......so lowest A.E....
must have been (b),ans (d) diya hai.......Now think of an alternate method??
Q1) ka ans (b) hoga..... becoz 3 deg carbo are more stable and less act energy ....
i guess the ans may be wrong....
but can u plz tell me abt Q2)... i can't got it ..!!!
For 2) the only thing that is cuming to my mind is of the strenght of acid........Akshay tum kya logic lagaye ike liye????