106
106
edit: the questions are 3,4,5,6 respectively
 19
19ans 3> (b) ...its a tertiary carbocation from its outset !
ans 4> (d)
 13
132) In 1 & 4, O & N pe +ve charge hai..resp, so they are eager to neutralise, Since the tendency 2 gain an electron of Oxygen > tht of Nitrogen, 1>4,
...n since a -ve charge is stabler on O than on Nitrogen, 3>2.
3) b)...'coz more alkylated alkenes are more reactive..
4) will go with d) too...
5) c) perhaps..
6) CH3-Cl -----(Zn/HCl)---> CH4
CH3-Cl ---i)(Mg/Ether)----ii)H3O+-----> CH4
 39
396) CH3 - Cl + aq KCN ---> CH3CN
CH3CN + H3O+ ----> CH3COOH
CH3COOH + NaOH/CaO ---> CH4
 13
13Arrey, lekin he says max. 2 Steps naa ??
 13
13Ek aur....
CH3-Cl ---(NaI/Acetone)---> CH3-I
CH3-I + HI <------> CH4 + I2 (But this one is highly reversible...so not a recommended one!)
 39
39Oh I read it as three lol
If its highly reversible, use Le Chatelier's principle to maximise yield na..pressure temperature mention kar de.
 106
106Q3. answer given (d)
Q4. (c)
Q5. (b) I have doubts differentiating betn b and d
Q6. options were given as (multi-answer)
(a) Zn/H+
(b) LiAlH4 (have doubt in this one)
(c) Mg/H20(ether) then H2O
(d) All
Q1. still unsolved
 106
106Q7. Compare the heats of combustion of the following compounds:
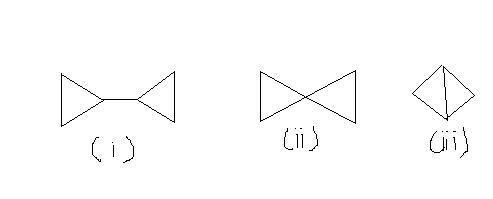
 3
3heats of combustion of the following compounds:
1>2>3
 11
11as the no of carbon is increasing in the order 1>2>3 so heat of combustion will also follows this order
 106
106thx.. swaraj
btw. we r going to have a new phy teacher who will teach physics exclusively for 1 week and also an inorganic teacher.. director sir said,,, btw enjoying in cuttack?
pls try the other questions
 11
115>>>>>As in phenol the lone pair of oxygen is also have some contribution in resonance so it have slightly +ve char.hence the H attached to this O has more affinity towards lone pair of others. that's why intermolecular H bonding is strongest in phenol
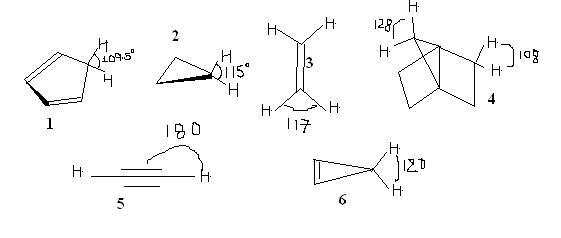
 3
3for the first one 1<5<4<3<2<6