I've noticed that recently not too many questions have come on this forum. You want AIEEE practice? You're gonna get it. Try this. I got it from Yahoo Answers(answered it myself there).
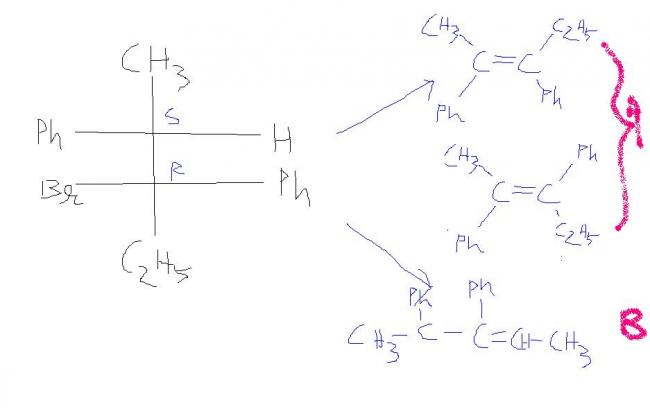
A chemist allows some pure (2S, 3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide (NaOCH2CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single pure isomer. Under the same conditions, the reaction of (2S, 3S)-3-bromo-2,3-diphenylpentane gives two alkenes, A (cis-trans mixture) and C. Upon catalytic hydrogenation, all three of these alkenes (A, B, and C) give 2,3-diphenylpentane. Determine the structures of A, B, and C, give equations for their formation, and explain the stereospecificity of these reactions. Pay careful attention to stereochemistry.
Now don't go running off to Y! Answers to get the readymade solution of this...try yourself first lol
-
UP 0 DOWN 0 0 3

3 Answers
If you could show those in sawhorse projection it would be more accurate(the mixture A). Meaning depict the mechanism in sawhorse projection.
B is fine!