B is isopropyl alcohol
A [12]
An Ester A(C4H8O2) on treatment with excess methyl chloride followed by acidification, gives an alcohol B as the sole organic product.
Alcohol B, on oxidation with NaOCl followed by acidification, gives acetic acid. Deduce the structure of A and B.
Show the racions involved.
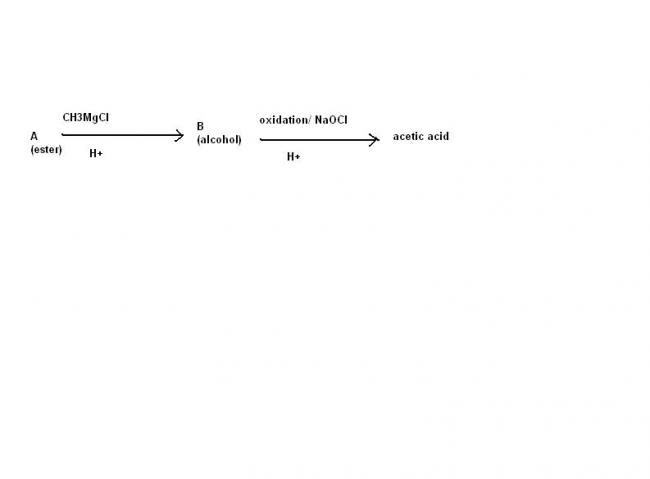
B shoukd be a primary or secondary alcohol
primary alcohol is not possible , secondary alcohol is possible if the ester is that of fromic acid
thus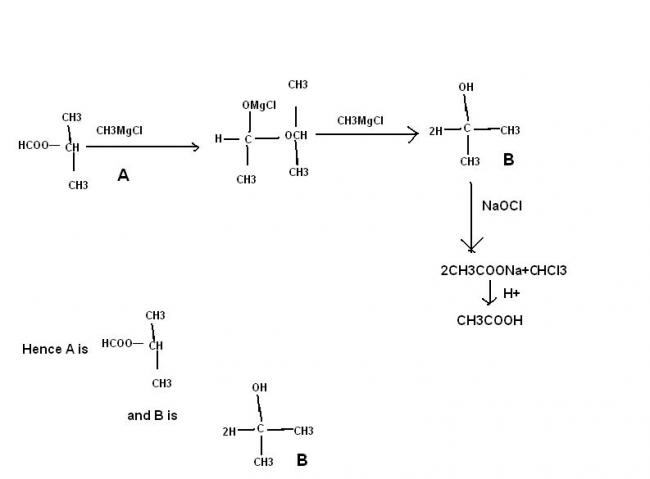
Sir, pl. give some hint or the first step........
its been a while for this questn now!!!
hey tapan the answers are correct but dunno of reaction b/w ester and the methyl chloride .
Sri da,
An Ester A(C4H8O2) on treatment with excess methyl chloride followed by acidification
Widout knowin this ^^^ how can v get da answer???
Alcohol B, on oxidation with NaOCl followed by acidification, gives acetic acid
see this gives the link for the alcohol and use the formula to get the acid
oh okie!!!
BUT lets HUNT for the reactn : ester + X's CH3Cl gives wat??
may be cl will attack on the carbon of carbonyl group and OC3H7 will depart