For 3, I too think there should be no elimination but then I'm not sure.
@Aditya - Thanks for the 5th one. My reasoning for 2 was same. :)
1. 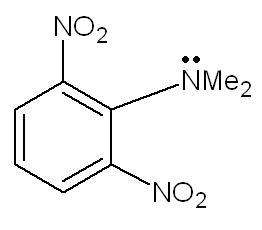
Unlike other aromatic amines, why is this amine strongly basic?
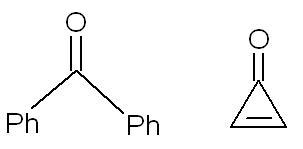
2. Compare the dipole moments of the following compounds:
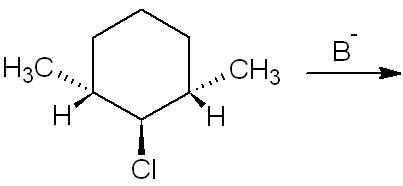
3. What is the major elimination product?
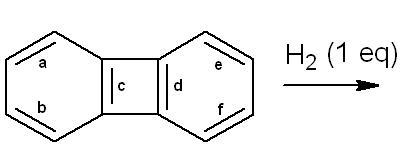
4.Identify the bond which will undergo hydrogenation:
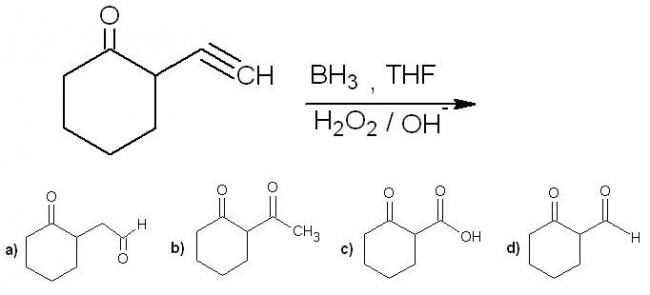
5. 
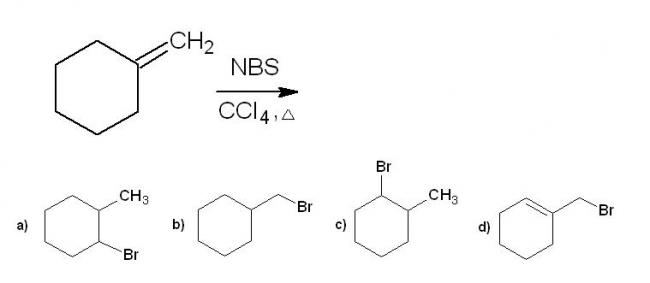
6. 
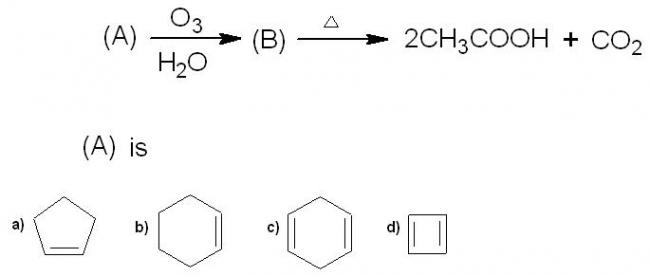
7. 
1. due to steric hindrance from - NO2 groups at ortho pos. , the N-Me2 group goes out of plane and hence cannot participate in resonance with the benzene ring. therefore lone pairs are readily available , hence strongly basic.
2. the second compund has more dipole moment as it leads to an aromatic ring which is more stable.
5.a) hydroboration - anti marakoni koff product. then enol to keto tautomerism.
3. i think no product will be formed since both H-atoms are parallel to the chlorine atom
4. look at aromaticity in this situation....i dont think hydrogenation shud take place...
chmistry is full of anomalies....so i`m nt sure....for the rest i hv to study them
For 3, I too think there should be no elimination but then I'm not sure.
@Aditya - Thanks for the 5th one. My reasoning for 2 was same. :)
For 4, somehow my answer doesn't match with any of the options given.
Btw, since I dont have the answers, we'll have to wait till some experts validates!
1) How strong acidity or basicity can be is found out by checking the stability of conjugate acids and bases. As Aditya said, there is a Steric Inhibition of Protonation (a kind of ortho effect) which causes NMe2 to go out of plane wrt the ring. This essentially terminates the resonance connection. If I protonate the amine group now, from where will the conjugate acid derive it's stability? There is no resonance...shouldn't this decrease the basicity of this compound instead? Coz that's what happens in the ortho effect for aniline type structures :\.
This clashes with what we're taught in school (how aniline is less basic than regular amines due to resonance of lone pair) but they never compared different aromatic amines, now, did they?
Agree with the answers for all the other questions. 7th one is quite easy...try the set of reactions on each option, you'll find your answer. Main thing to remember here is that this is ozonolysis with oxidative workup and not reductive(DMS) workup.
@ nasiko - what is the diff. between the c and d bonds in Q.4 ?
i believe both are purely identical.
That compound is called biphenylene. It is formed when 2 mols of benzyne combine together and is hence a dimer of benzyne. Since biphenylene is anti-aromatic (it only appears planar), I guess on hydrogenation of c it becomes planar, which is why nasiko answered c...
i think the answr to the question on hydrogenation is c...as the other two rings r aromatic n hence extremely stable...
d consequence is thus obvious...:)
what is d ans given???
My doubt is similar to Aditya.. Arent the two bonds (c and d) exactly identical?
i think the product which will be formed is not given in the options....
For question 6, the product (A) is the option (d). This is due to radical stabilisation .
i think that will be the minor product....the major product is something else....