gagar then major one will be one with five membered ring no ?
WAT ARE THE POSSIBLE METHODS OF convertin HOMOGENOUS N CARBON ATOMed RING TO (N+1) CARBON ATOMed RING???
-
UP 0 DOWN 0 0 38

38 Answers
and bhiyya if i do the same with a 3 member ring will it expand ????????
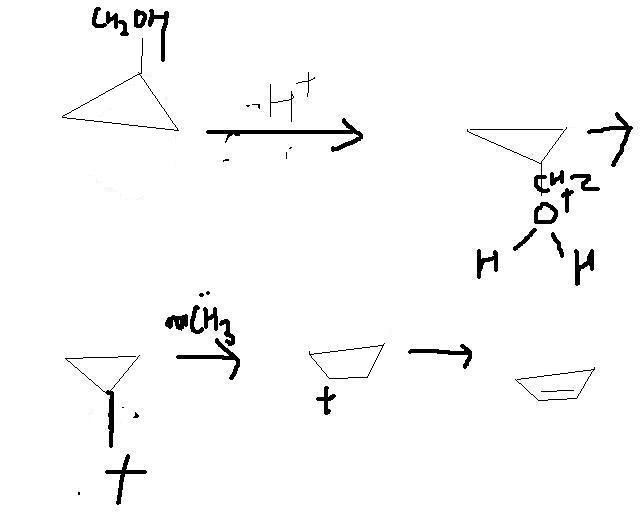
cuz i dont see exceptional stability in 3 member ring like the 6 member 1!!!!!![2][2]
heeeelllllllllllpppppppppppppppp pme bhiyyaaaaa!!!!!!!!!!!!!!!!!!!!!![2][2]2[2][2][2][2][2]
but the strain reduces a lot..
see here the carbon is sp3 hybridised.
so the bond angle should be 108-9 degree..
but the geometry causes it to be at 60!
so there is a lto fo strain.
In going to a 4 carbon ring structure most of the strain is gone..
so there is huge energy released and thus more stability.

This is what I think should have happened in that case iitimcoming..
your analysis for the 3 membered structure seems correct
@ nishant i am also thinking of any shortcut method for this conversion but not getting any although by breaking the ring and then using LDA for directed aldol would bring the seven membered ring
if u got any shorter one plz let me know
hmm.. okie..
you expect me to know that even when you dont.. ;)
but in case (wich is most unlikely.. ) if i do.. i will try to..
im unable to understand how to make it a 3° carbocation....pls help[2][2][2][2]
SRINATH THATS WHY I AM USING LDA FOR DIRECTED ALDOL THIS WILL GIVE SEVEN MEMBERED RING EXCLUSIVELY (i am using the word exclusive b'cos in case of LDA we form only one product)
bhaiya in pinacol-pinacolone there we can have ring expansion.....
well tapan this method can be used here try to bring NH2 grp at the methyl thing then demayjanov ring expansion I dunno abt yield seven membered ring no?
n=6
ha ha....
no sky actually jus wanted to 4 usage in conversions this aint question frm a paper or sumthin [1]
but ya no. of reactions can get clarified if v solv this.....
hmm..
din get ur question fully...
then also...
1) do chlorination in pr of light.
2) Ph- CH2Cl formed.
3) now , anyhow remove Cl-... so carbocation formed...
4) here ring may expand...
but it wont .. coz 6-mem ring is very very vey stable...
@ vishal , carbene I think can be used but that places a lot of restriction on the substituents I think .
sri nothing much is specified i just gave a general methaod to step up but yes u r somewhat rite